经典古方开新散的慢性口服毒性评价。
IF 4.8
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
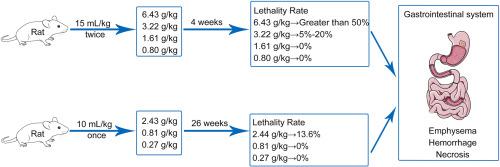
民族药理学意义:开窍散(KXS)作为古代经典处方,用于治疗健忘症已有数千年的历史。现代临床和非临床药理研究发现,它对痴呆症和抑郁症有显著的治疗效果,但有关其安全性的研究相对较少:研究目的:进行亚急性和慢性毒性研究,以调查毒性反应的症状、严重程度、靶器官、发展和恢复情况以及毒性剂量。这些研究为确保 KXS 的安全性提供了技术数据:在亚急性毒性研究中,给大鼠口服 KXS,剂量分别为 0.80、1.61、3.22 和 6.43 克/千克体重,持续 4 周。在慢性毒性研究中,按每公斤体重 0.27、0.81 和 2.43 克的剂量给大鼠口服 KXS,持续 26 周,并在治疗后进行为期 4 周的停药研究。每天观察大鼠的临床症状和死亡情况,定期监测体重、食量和饮水量的变化。此外,还在特定的观察时间点监测尿液分析结果、血液学和生化参数、相对器官重量和病理变化:结果:在亚急性毒性研究中,对死亡和病死大鼠的尸体解剖显示,胃肠道明显膨胀和肿胀,肠壁变薄。观察到的主要不良反应包括胀气、皮疹、呼吸声异常和消瘦。1.61 克/千克及以下的剂量不会导致动物死亡。胃肠道系统是毒性的主要靶器官。在慢性毒性研究中,KXS 的无观测不良效应水平(NOAEL)为 0.27 克/千克,其毒性作用主要集中在胃肠系统。这导致了免疫系统、造血系统和心脏的继发性病理变化,表明在临床上长期使用大剂量时应监测相关指标:结论:在啮齿动物毒性评估过程中,给大鼠服用含有粗制药物粉末的 KXS 会造成严重的胃肠道损伤。大鼠的无观测不良效应水平为 0.27 克/千克/天。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Evaluation of the chronic oral toxicity of the classical ancient prescription Kai-Xin-San
Ethnopharmacological relevance
The Kai-Xin-San (KXS), as an ancient classic prescription, has been used for the treatment of amnesia for thousands of years. Modern clinical and non-clinical pharmacological studies have found that it has significant therapeutic effects on dementia and depression, but there are relatively few studies on its safety.
Aim of the study
Subacute and chronic toxicity studies were conducted to investigate the symptoms, severity, target organs, development and recovery of toxic reactions, as well as the toxic dose. These studies provide technical data for ensuring the safety of KXS.
Materials and methods
In the sub-acute toxicity study, rats were orally administered KXS at doses of 0.80, 1.61, 3.22, and 6.43 g/kg body weight for a duration of 4 weeks. In the chronic toxicity study, rats were orally administered KXS at doses of 0.27, 0.81, and 2.43 g/kg body weight for a duration of 26 weeks, and a withdrawal study was conducted for a period of 4 weeks after the treatment.The rats were observed daily for clinical signs and mortality. Changes in body weight, food consumption, and water consumption were periodically monitored. Additionally, urinalysis results, hematological and biochemical parameters, relative organ weights, and pathology were monitored at specific observation time points.
Results
In the sub-acute toxicity study, necropsy of dead and moribund rats revealed evident distension and swelling of the gastrointestinal tract, as well as thinning of the intestinal wall. The main adverse reactions observed included flatulence, piloerection, abnormal breathing sounds, and emaciation. Doses of 1.61 g/kg and below did not cause animal death. The gastrointestinal system is the main target organ of toxicity. In the chronic toxicity study, the no-observed-adverse-effect-level (NOAEL) of KXS was 0.27 g/kg, and its toxic effects were primarily concentrated in the gastrointestinal system. This led to secondary pathological changes in the immune system, hematopoietic system, and heart, suggesting that relevant indicators should be monitored when large doses are used clinically for an extended period of time.
Conclusions
During the rodent toxicity evaluation, severe gastrointestinal damage was observed when KXS, powdered with crude drugs, was administered. The NOAEL for rats was found to be 0.27 g/kg/day.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of ethnopharmacology
医学-全科医学与补充医学
CiteScore
10.30
自引率
5.60%
发文量
967
审稿时长
77 days
期刊介绍:
The Journal of Ethnopharmacology is dedicated to the exchange of information and understandings about people''s use of plants, fungi, animals, microorganisms and minerals and their biological and pharmacological effects based on the principles established through international conventions. Early people confronted with illness and disease, discovered a wealth of useful therapeutic agents in the plant and animal kingdoms. The empirical knowledge of these medicinal substances and their toxic potential was passed on by oral tradition and sometimes recorded in herbals and other texts on materia medica. Many valuable drugs of today (e.g., atropine, ephedrine, tubocurarine, digoxin, reserpine) came into use through the study of indigenous remedies. Chemists continue to use plant-derived drugs (e.g., morphine, taxol, physostigmine, quinidine, emetine) as prototypes in their attempts to develop more effective and less toxic medicinals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


