线粒体功能失调引发的亮德细胞早衰部分参与了 1-硝基芘诱发的睾丸类固醇合成酶下调。
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
过去几十年来,男性血清睾酮(T)一直在下降。之前的报告发现,1-硝基苯芘(1-NP)暴露会抑制睾丸睾酮的合成。本研究旨在进一步探讨早衰是否参与了 1-NP 触发的睾丸 T 合成减少。成年雄性小鼠每天口服 1-NP(0、100 和 500 μg/kg)14 天。服用 1-NP 的小鼠血清和睾丸中的 T 含量均减少。位于线粒体的类固醇合成酶(包括 StAR、CYP11A1 和 3βHSD1 )在服用 1-NP 的小鼠睾丸和 MLTC-1 细胞中下调。从机理上讲,1-NP 暴露通过抑制依赖于 NAD+ 的去乙酰化酶 SIRT3 的酶活性,增加了线粒体类固醇合成酶的乙酰化修饰。补充 NAD + 前体和过表达 Sirt3 可缓解 1-NP 触发的小鼠睾丸和 MLTC-1 细胞中类固醇合成酶水平的降低。相反,沉默 Sirt3 会加剧 1-NP 引起的乙酰化和 MLTC-1 细胞中类固醇合成酶水平的降低。进一步的实验证明,暴露于 1-NP 会导致小鼠睾丸和 MLTC-1 细胞线粒体功能失调和过早衰老。补充线粒体定向抗氧化剂线粒体醌(MitoQ)可防止 1-NP 引起的睾丸细胞过早衰老和睾丸类固醇合成酶的下调。这些结果表明,线粒体功能失调引发的Leydig细胞早衰可能部分参与了1-NP诱发的睾丸类固醇合成酶水平的降低。本文章由计算机程序翻译,如有差异,请以英文原文为准。
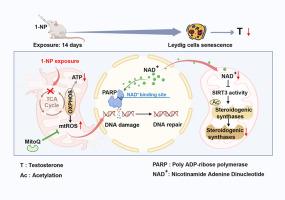
Mitochondrial malfunction-initiated Leydig cell premature senescence partially participates in 1-nitropyrene-evoked downregulation of steroidogenic synthases in testes
Serum testosterone (T) in males has been declining during the past decades. The previous reports found that 1-nitropyrene (1-NP) exposure suppressed testicular T synthesis. The purpose of the current study was to further explore whether premature senescence participates in 1-NP-triggered reduction of testicular T synthesis. Adult male mice were orally exposed to 1-NP (0, 100, and 500 μg/kg) daily for 14 days. Serum and testicular T contents were diminished in 1-NP-administered mice. Mitochondria-located steroidogenic synthases, including StAR, CYP11A1, and 3βHSD1, were downregulated in 1-NP-administered mouse testes and MLTC-1 cells. Mechanistically, 1-NP exposure increased acetylation modification of mitochondrial steroidogenic synthases by inhibiting the enzymatic activity of SIRT3, an NAD+-dependent deacetylase. Supplementing NAD + precursor and Sirt3 overexpression relieved 1-NP-triggered reduction of steroidogenic synthase levels in mouse testes and MLTC-1 cells. By contrast, Sirt3 silencing aggravated 1-NP-evoked acetylation and reduction of steroidogenic synthase levels in MLTC-1 cells. Further experiments demonstrated that 1-NP exposure caused mitochondrial malfunction and premature senescence in mouse testes and MLTC-1 cells. Supplementation with mitochondria-directed antioxidant mitoquinone (MitoQ) prevented 1-NP-evoked Leydig cell premature senescence and downregulation of testicular steroidogenic synthases. These results suggest that mitochondrial malfunction-initiated Leydig cell premature senescence may partially participate in 1-NP-evoked reduction of steroidogenic synthase levels in testes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


