间充质基质细胞通过细胞外囊泡输送H2S增强的Nrf2,以介导线粒体平衡,从而修复缺氧缺血性脑损伤。
IF 7.1
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
间充质基质细胞(MSCs)被认为是通过细胞外囊泡(EVs)治疗神经系统疾病的一种方法。经过修饰的EVs含有活性成分,具有更高的治疗潜力。在这项研究中,我们旨在探索经 NaHS(一种 H2S 供体)预处理的间充质干细胞的 EVs(H2S-EVs)在缺氧缺血(HI)脑损伤中的作用和基本机制。我们的研究结果表明,通过非侵入性鼻内途径对HI小鼠进行H2S-EVs治疗与EVs治疗相比,能够减轻氧化应激和线粒体功能障碍。机理研究表明,NaHS通过诱导间充质干细胞中帕金森病蛋白7(PARK7)依赖性的Nrf2/Keap-1复合物解体,促进了细胞质中核因子红细胞-2相关因子2(Nrf2)的表达。特别是,游离的Nrf2被载入EVs,这是由于其KFERQ基序被70-kDa热休克蛋白和溶酶体相关膜蛋白2A识别。随后,H2S-EVs 被内化到同侧大脑半球的神经元中,从而将丰富的 Nrf2 运送到线粒体中,并在 HI 小鼠经 H2S-EVs 处理后重塑线粒体功能。此外,敲除间充质干细胞中的Nrf2会显著削弱H2S-EVs对HI小鼠的治疗效果。简而言之,本研究首次证明了H2S修饰的间充质干细胞通过上调PARK7的表达,在EVs中积累了较高的Nrf2,揭示了H2S-EVs提供的抗氧化蛋白Nrf2保护HI脑损伤线粒体功能障碍的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
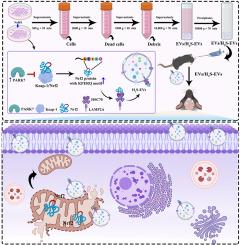
Mesenchymal stromal cells deliver H2S-enhanced Nrf2 via extracellular vesicles to mediate mitochondrial homeostasis for repairing hypoxia-ischemia brain damage
Mesenchymal stromal cells (MSCs) are considered a therapeutic approach for neurological diseases via extracellular vesicles (EVs). Modified EVs contain active components with enhanced therapeutic potential. In this study, we aimed to explore the role and underlying mechanism of EVs from MSCs preconditioned by NaHS (an Hydrogen sulfide donor) (H2S-EVs) in hypoxia-ischemia (HI) brain damage. Our results showed that H2S-EVs treatment via the non-invasive intranasal route in HI mice was able to reduce oxidative stress and mitochondrial dysfunction compared to EVs treatment. Mechanistic studies demonstrated that NaHS promoted nuclear factor erythroid-2 related factor 2 (Nrf2) expression in the cytoplasm by inducing Parkinson disease protein 7 (PARK7)‐dependent disintegration of Nrf2/Keap-1 complex in MSCs. In particular, the free Nrf2 was loaded into the EVs as a result of its KFERQ motif being recognized by 70-kDa heat shock proteins and lysosomal-associated membrane protein 2A. Subsequently, H2S-EVs were internalized into neurons in the ipsilateral hemisphere, thus delivering abundant Nrf2 to accumulate in the mitochondria and remodeling mitochondrial function following H2S-EVs treatment in HI mice. Moreover, Nrf2 knockdown in MSCs remarkably impaired H2S-EVs-mediated therapeutic effects on HI mice. In brief, the present study for the first time demonstrated that H2S-modified MSCs significantly accumulated higher Nrf2 in EVs via upregulating PARK7 expression, revealing the mechanism through which antioxidant protein Nrf2 delivered by H2S-EVs protect against mitochondrial dysfunction in HI brain damage.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


