过氧化物酶 1 或过氧化物酶 2 基因敲除对 Jurkat 细胞硫醇蛋白质组的影响
IF 7.1
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
过氧化还原酶是细胞过氧化物代谢的重要调节剂。作为抗氧化剂,它们能限制其他细胞蛋白的氧化,但作为信号分子,它们又能充当传感器,通过氧化还原中继机制促进硫醇蛋白氧化。因此,过氧化还原酶的存在可能会影响其他硫醇蛋白,即使在具有内源性氧化还原活性的细胞中也是如此。为了对两种细胞质过氧化还原蛋白 Prdx1 和 Prdx2 进行研究,我们比较了野生型 Jurkat 细胞与敲除其中一种过氧化还原蛋白的细胞的硫醇蛋白质组。通过质谱分析和同位素标记,我们在WT/KO的比较中检测到了大约10,000个常见的含CysSH的肽段。Prdx1或Prdx2的敲除导致一小部分Cys残基的氧化还原状态发生变化,不到100个Cys残基的氧化还原状态差异超过2倍。引人注目的是,其中很大一部分,包括那些变化最大的残基,在两种 KO 中都是共通的。一些 Cys 残基在基因剔除物中的氧化程度较高,而另一些则较低。候选蛋白质的功能多种多样,对氧化剂并不敏感。其他 Prdxs 和氧化剂敏感蛋白的 Cys 残基的氧化还原状态没有差异。在 Prdx2 基因敲除细胞中,有 7 种细胞骨架或调节性硫醇蛋白的表达发生了变化,其中 3 种已通过 Western 印迹法进行了测试和验证。几乎没有确凿证据表明硫醇氧化还原变化依赖于任何一种 Prdx,而这种变化可通过中继机制归因于氧化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
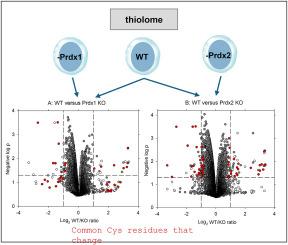
Effect of peroxiredoxin 1 or peroxiredoxin 2 knockout on the thiol proteome of Jurkat cells
Peroxiredoxins are important regulators of cellular peroxide metabolism. As antioxidants, they restrict oxidation of other cell proteins, but as signaling molecules they can act as sensors and promote thiol protein oxidation via a redox relay mechanism. The presence of peroxiredoxins could therefore influence other thiol proteins, even in cells experiencing endogenous redox activity. To investigate this for the two cytoplasmic peroxiredoxins, Prdx1 and Prdx2, we have compared the thiol proteome of wildtype Jurkat cells with cells in which either one was knocked out. Using mass spectrometry and isotope tagging, approximately 10,000 common CysSH-containing peptides were detected for each WT/KO comparison. Knockout of Prdx1 or Prdx2 resulted in a change in redox state of a small selection of Cys residues, with less than 100 giving more than a 2-fold difference. Strikingly, a large proportion of these, including those that showed the greatest change, were common to both KOs. Some Cys residues showed more oxidation in the knockouts, whereas others showed less. The candidate proteins have diverse functions and have not been known to be oxidant sensitive. No differences were seen in redox state of Cys residues of other Prdxs and oxidant sensitive proteins. A change in expression in Prdx2 knockout cells was indicated for seven cytoskeletal or regulatory thiol proteins, three of which were tested and validated by western blotting. Little firm evidence was found for thiol redox changes dependent on either Prdx that could be attributed to oxidation via a relay mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


