在神经外科手术中应用热响应糊剂中的奥拉帕利可增强 DNA 损伤,从而延长恶性胶质瘤的存活时间。
IF 6.4
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
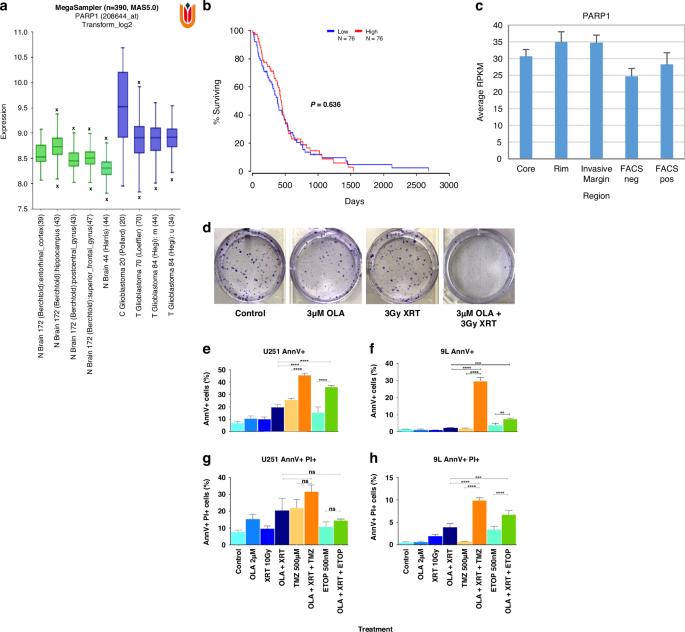
背景:人们越来越关注将多腺苷二磷酸核糖聚合酶-1(PARP-1)抑制剂奥拉帕利重新用于新诊断或复发的异柠檬酸脱氢酶野生型胶质母细胞瘤。我们探讨了在临床前高级别胶质瘤模型中腔内给药奥拉帕利是否会带来生存益处:方法:利用原发肿瘤 RNA 测序数据确定 PARP-1 为胶质母细胞瘤浸润边缘的靶点。我们利用克隆生长试验、细胞周期分析和免疫细胞化学,在体外评估了奥拉帕利单独或与基因毒性损伤同时使用所产生的放射增敏作用,并在体内评估了手术后通过温度敏感的聚合糊剂给药所产生的放射增敏作用:结果:RNA测序证实PARP-1是胶质母细胞瘤浸润性疾病的可行治疗靶点。神经胶质瘤细胞急性暴露于奥拉帕利会影响增殖,并诱导体外与DNA损伤相关的晚期凋亡,辐射会增强这种作用。利用高级别胶质瘤正位异位移植,观察到与全身性奥拉帕利和标准胶质母细胞瘤治疗相比,在放疗的同时进行奥拉帕利间质给药可获得长期的总生存期。奥拉帕利与替莫唑胺或依托泊苷联合给药可提高长期生存率,这表明奥拉帕利具有DNA损伤增敏剂的功能:总之,我们的数据支持在目前治疗恶性胶质瘤的临床方案中同时使用局部奥拉帕利。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Neurosurgical application of olaparib from a thermo-responsive paste potentiates DNA damage to prolong survival in malignant glioma
There is increased pan-cancer specific interest in repurposing the poly adenosine diphosphate-ribose polymerase-1 (PARP-1) inhibitor, olaparib, for newly diagnosed or recurrent isocitrate dehydrogenase wild type glioblastoma. We explore whether intra-cavity delivery of olaparib confers a survival benefit in a pre-clinical high-grade glioma model. Primary tumor RNA sequencing data was used to determine PARP-1 as a target in the glioblastoma infiltrative margin. We assessed radiosensitization conferred by olaparib alone and concomitant to genotoxic insults in vitro using clonal growth assays, cell cycle analysis and immunocytochemistry, and in vivo upon post-surgical delivery from a temperature-sensitive polymeric paste. RNA-sequencing confirmed PARP-1 as a viable therapy target in glioblastoma infiltrative disease. Acute exposure of glioma cells to olaparib impaired proliferation and induced late-stage apoptosis associated with DNA damage in vitro, potentiated by radiation. Using high-grade glioma orthotopic allografts, a long-term overall survival benefit was observed upon interstitial olaparib delivery concomitant with radiotherapy, compared to systemic olaparib and standard glioblastoma treatment. Combined delivery of olaparib with either temozolomide or etoposide increased long-term survival, suggestive of olaparib functioning as DNA damage sensitizer. Collectively, our data support a rationale for localized olaparib delivery concomitant with the current clinical regimen for malignant glioma treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

British Journal of Cancer
医学-肿瘤学
CiteScore
15.10
自引率
1.10%
发文量
383
审稿时长
6 months
期刊介绍:
The British Journal of Cancer is one of the most-cited general cancer journals, publishing significant advances in translational and clinical cancer research.It also publishes high-quality reviews and thought-provoking comment on all aspects of cancer prevention,diagnosis and treatment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


