熊去氧胆酸通过 FXR 抑制 p38 MAPK/NF-κB 信号通路,减轻脂肪栓塞综合征诱发的急性肺损伤。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
脂肪栓塞综合征(FES)引起的急性肺损伤(ALI)是一种死亡率很高的疾病。本研究旨在探讨熊去氧胆酸(UDCA)在脂肪栓塞综合征诱发的急性肺损伤中的作用及其内在机制。通过异体移植小鼠肾周脂肪,建立了 ALI 小鼠模型。在体外实验中,用脂肪酸处理人肺微血管内皮细胞(HPMEC)。研究了 UDCA 对内皮细胞中法尼类固醇 X 受体(FXR)表达和炎症反应的影响。在 FES 诱导的 ALI 中,UDCA 能明显抑制炎症反应和促炎症标志物的表达。UDCA 可明显降低体外 TNF-α 和 IL-1β 的表达。服用 UDCA 能显著上调 FXR 的表达,并显著降低 p38 MAPK 和 NF-κB p65 的磷酸化。敲除 FXR 的表达会降低 UDCA 在体内的作用。此外,在体外敲除 FXR 表达和过表达 FXR 分别增加和减少了炎症反应。此外,服用 p38 MAPK 激活剂可逆转 FXR 过度表达的抗炎作用。UDCA 通过抑制 p38 MAPK/NF-κB 信号传导和激活 FXR 改善了 FES 诱导的 ALI 期间的炎症反应。这些发现为 UDCA 治疗 FES 诱导的 ALI 的潜力提供了新的证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
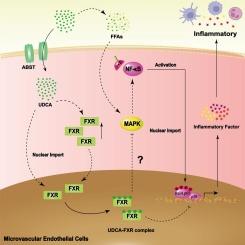
Ursodeoxycholic acid alleviates fat embolism syndrome-induced acute lung injury by inhibiting the p38 MAPK/NF-κB signalling pathway through FXR
Acute lung injury (ALI) caused by fat embolism syndrome (FES) is a disease with high mortality. This study aimed to explore the roles of ursodeoxycholic acid (UDCA) in FES-induced ALI and its underlying mechanisms. An ALI mouse model was established by allografting mouse perinephric fat. For in vitro experiments, human pulmonary microvascular endothelial cells (HPMEC) were treated with FFAs. The effects of UDCA on the expression of farnesoid X receptor (FXR) and the inflammatory response in endothelial cells were investigated. UDCA significantly inhibited the inflammatory response and the expression of proinflammatory markers during FES-induced ALI. UDCA markedly decreased TNF-α and IL-1β expression in vitro. UDCA administration markedly upregulated FXR expression and significantly reduced the phosphorylation of p38 MAPK and NF-κB p65. Knock down FXR expression decreased the effect of UDCA in vivo. Furthermore, knock down FXR expression and overexpressing FXR increased and decreased the inflammatory response, respectively, in vitro. Moreover, administration of a p38 MAPK activator reversed the anti-inflammatory effect of FXR overexpression. UDCA ameliorated inflammation during FES-induced ALI by suppressing p38 MAPK/NF-κB signalling and activating FXR. These findings provide new evidence for the potential of UDCA for FES-induced ALI treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


