泊纳替尼通过PDGFR-PI3K/AKT通路加剧系统性红斑狼疮小鼠模型的肾损伤
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
狼疮性肾炎(LN)是系统性红斑狼疮(SLE)常见的临床并发症。增殖性狼疮性肾炎是狼疮性肾炎中最严重的一种,由于目前仍缺乏有效的治疗药物,酪氨酸激酶抑制剂(TKIs)因其在抑制细胞增殖方面的显著作用而被广泛应用于临床,并可能成为治疗狼疮性肾炎的潜在药物。然而,以往关于 TKI 对 LN 影响的研究一直存在争议。作为第三代 TKI,泊纳替尼缺乏对其在 LN 中作用的研究。本研究旨在探讨泊纳替尼对LN的影响。口服泊纳替尼后,对MRL/lpr小鼠的肾功能、自身免疫标记物和组织病理学变化进行了评估。进行了RNA-seq分析,以探索参与泊纳替尼诱导的肾损伤的分子通路。在不影响狼疮相关自身免疫标记物的情况下,泊纳替尼独特地加剧了MRL/lpr小鼠的肾损伤,表现为肾功能下降和急性病理变化。差异表达基因分析和功能富集表明,泊纳替尼诱导的MRL/lpr小鼠肾损伤与脂联素有关。此外,我们还验证了泊纳替尼通过PDGFRα向PI3K/AKT通路发出信号,可能会影响高分子量脂联素(HMW ADIPOQ)的表达并加剧肾损伤。总之,本研究表明,泊纳替尼可以通过抑制PDGFRα磷酸化,通过PI3K/AKT通路上调HMW ADIPOQ的表达,突出了泊纳替尼对狼疮易感小鼠的潜在肾毒性作用,并强调了监测接受泊纳替尼治疗的系统性自身免疫性疾病患者肾功能的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
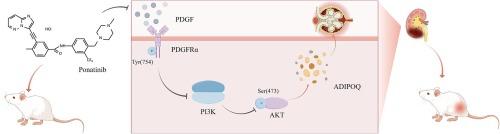
Ponatinib exacerbate renal injury in systemic lupus erythematosus mouse model through PDGFR-PI3K/AKT pathway
Lupus nephritis (LN) is a common clinical complication of systemic lupus erythematosus (SLE). Proliferative lupus nephritis represents the gravest form of LN, and since effective drugs for its treatment are still lacking, tyrosine kinase inhibitors (TKIs) find extensive clinical utility due to their notable impact on suppressing cell proliferation and may serve as potential drugs for LN treatment. However, previous studies on the effects of TKI on LN have been controversial. Ponatinib, a third-generation TKI, lacks studies on its role in LN. This study aimed to investigate the impact of the ponatinib on LN. MRL/lpr mice were evaluated for renal function, autoimmune markers and histopathological changes after oral administration of ponatinib. RNA-seq analysis was performed to explore the molecular pathways involved in ponatinib-induced kidney injury. Ponatinib uniquely exacerbated renal damage in MRL/lpr mice, evidenced by a decline in renal function and acute pathological changes, without affecting lupus-related autoimmune markers. Differential expressed genes analysis and functional enrichment implicate ponatinib-induced renal damage in MRL/lpr mice associated with adiponectin. Furthermore, we verified ponatinib signaling the PI3K/AKT pathway through PDGFRα, potentially influencing high molecular weight adiponectin (HMW ADIPOQ) expression and exacerbating renal damage. In conclusion, this study demonstrates that ponatinib can up-regulate HMW ADIPOQ expression via the PI3K/AKT pathway by inhibiting PDGFRα phosphorylation, highlighting the potential nephrotoxic effects of ponatinib in lupus-prone mice, and underscoring the importance of monitoring renal function in systemic autoimmune diseases patients receiving ponatinib.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


