通过固定假单胞菌脂肪酶提高鼠李糖脂的生产:比较研究
IF 4.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
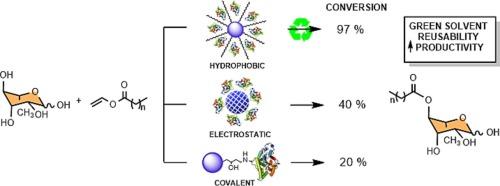
鼠李糖脂(RLs)具有出色的表面活性、乳化特性和生物活性,是一种被广泛研究的生物表面活性剂,在化妆品、制药和生物修复领域具有巨大的工业潜力。然而,高昂的生产成本阻碍了它们的大规模生产。本研究通过鼠李糖与月桂酸乙烯酯化反应酶法合成 4-O-lauroylrhamnose 的过程,重点研究了鼠李糖与月桂酸乙烯酯化反应酶法合成 4-O-lauroylrhamnose 的产率和区域选择性。比较了三种固定化方法:共价结合、在 Celite 上的吸附和在疏水支持物上的吸附。所使用的方法不同,固定效率也不同,在 Celite 上的吸附效率最低(56%),其次是在 Sepabeads 上的共价固定(EC-EP/S 78% 和 EC-EP/L 70%),而在疏水支持物上的吸附效率最高(83-97%,其中 EC-OD 的吸附效率最高,为 97%)。对于 4-O-lauroylrhamnose 的酶法合成,由于酶的构象自由度受限,共价固定在 Sepabeads™ EC-EP 上的转化率较低。Celite® 545 吸附法的转化率适中,但静电作用限制了酶的活性。疏水性支持物,特别是 Purolite® ECR8806F 的结果最有希望,在四氢呋喃和绿色溶剂 2-甲基四氢呋喃(2-MeTHF)中几乎实现了完全转化,并在鼠李糖的 4 位保持了高区域选择性。该研究强调了支持物疏水性和活性表面积在固定化酶性能中的关键作用。固定在 Purolite® ECR8806F 上的 PSL 显示了可持续生产 RLs 的巨大潜力,在多个反应循环中表现出卓越的可重复使用性、稳定性和生产率。这项研究通过优化 PSL 的固定化和反应条件,在 RLs 生产方面取得了重大进展,为更具成本效益和可持续的工业应用提供了便利。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhancing rhamnolipid production via immobilized Pseudomonas stutzeri lipase: A comparative study
Rhamnolipids (RLs) are widely studied biosurfactants with significant industrial potential in cosmetics, pharmaceuticals, and bioremediation due to their excellent surface activity, emulsifying properties and bioactive characteristics. However, high production costs impede their mass production. This study investigates the immobilization of Pseudomonas stutzeri lipase (PSL) on various supports to enhance RL synthesis efficiency, focusing on yield and regioselectivity in the enzymatic synthesis of 4-O-lauroylrhamnose by the transesterification of rhamnose with vinyl laurate. Three immobilization methods were compared: covalent binding, adsorption on Celite, and adsorption on hydrophobic supports. The immobilization efficiency varied depending on the method used, with the lowest observed for adsorption on Celite (56 %), followed by covalent immobilization on Sepabeads (EC-EP/S 78 % and EC-EP/L 70 %), and the highest for adsorption on hydrophobic supports (83–97 %, with EC-OD being the best at 97 %). For the enzymatic synthesis of 4-O-lauroylrhamnose, covalent immobilization on Sepabeads™ EC-EP yielded low conversions due to restricted conformational freedom of the enzyme. Celite® 545 adsorption resulted in moderate conversion rates, limited by the electrostatic interactions restricting enzyme activity. The most promising results were obtained with hydrophobic supports, particularly Purolite® ECR8806F, achieving nearly complete conversion and maintaining high regioselectivity at the 4-position of rhamnose in both THF and the green solvent 2-methyltetrahydrofuran (2-MeTHF). The study highlights the critical role of support hydrophobicity and active surface area in the immobilized enzyme performance. PSL immobilized on Purolite® ECR8806F demonstrated significant potential for sustainable RLs production, showing excellent reusability, stability and productivity across multiple reaction cycles. This study presents a significant advancement in RLs production by optimizing PSL immobilization and reaction conditions, facilitating the way for more cost-effective and sustainable industrial applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


