作为 NLRP3 炎症小体抑制剂的磺酰脲衍生物的设计、合成和生物学评价。
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
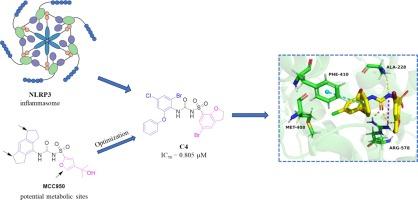
近年来,人们对 NLRP3 炎症小体进行了广泛研究,它的异常激活会加剧炎症反应,导致各种疾病。磺脲类药物 MCC950 是一种强效的 NLRP3 炎性体选择性抑制剂。然而,该药物的临床开发因肝毒性而停止,研究表明其代谢产物的活性显著降低。在 MCC950 的基础上,我们参考 NP3-146 的替代位点进行结构修饰,旨在解决潜在的代谢相关问题。因此,我们合成了一系列磺酰脲类衍生物。最终,优化后的化合物 C4 在 2 μM 的浓度下对 IL-1β 的抑制率高达 80.39%,IC50 值为 0.805 μM。总之,化合物 C4 显示出作为先导化合物的潜力,值得作为抗炎 NLRP3 抑制剂进一步开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design, synthesis and biological evaluation of sulfonylurea derivatives as NLRP3 inflammasome inhibitors
The NLRP3 inflammasome has been extensively studied in recent years and its aberrant activation can exacerbate inflammatory responses, contributing to various diseases. MCC950, a sulfonylurea drug, is a potent selective inhibitor of the NLRP3 inflammasome. However, its clinical development was halted due to hepatotoxicity, and studies have indicated significant reduction in activity among its metabolites. Building upon MCC950, we referenced substitution sites of NP3-146 for structural modifications aimed at addressing potential metabolism-related issues. Consequently, we synthesized a series of sulfonylurea derivatives. Ultimately, the optimized compound C4 exhibited a remarkable 80.39 % inhibition of IL-1β at 2 μM, with an IC50 value of 0.805 μM. In conclusion, compound C4 shows potential as a lead compound and warrants further development as an anti-inflammatory NLRP3 inhibitor.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


