一对新发现的多肽之间的选择性 pH 响应共轭作用
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
人们需要以刺激响应的方式实现完全可逆的位点选择性肽/蛋白质共轭化学。目前的定点选择性蛋白质修饰方法可以制备均一的蛋白质-小分子共轭物,这些共轭物在药物输送和化学生物学方面是不可或缺的,但这类化学反应通常是不可逆的。相比之下,现有的可逆蛋白质标记技术通常不具有位点选择性。在这里,我们报告了通过 mRNA 展示从头发现一对肽的方法,这对肽选择性地相互反应形成一种共轭物,这种共轭物在中性条件下(pH 值为 7.5)稳定,但在 pH 值为 10 时会迅速解离成成分。肽 CP1 中的一个 Cys 硫醇会迅速(k1 = 340 M-1-s-1)与伴侣 ITC6 中的异硫氰酸基发生反应,生成稳定的二硫代氨基甲酸酯(pH 值为 7.5 时,t1/2 = 6 小时)。我们的研究表明,该反应的 pH 响应特性(在 pH 值为 10 时,共轭物的 t1/2 = 5 分钟)可用于利用 ITC6 (1) 作为下拉手柄,从细胞裂解液中选择性地分离 CP1;(2) 作为临时保护基团,保护 CP1 不受碘乙酰胺等非特异性 Cys 标记试剂的影响。总之,这里开发的化学方法是对现有方法的补充,适用于需要选择性可逆反应的各种化学生物学环境。本文章由计算机程序翻译,如有差异,请以英文原文为准。
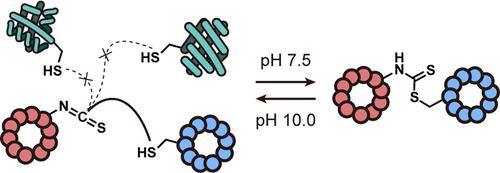
Selective pH-Responsive Conjugation between a Pair of De Novo Discovered Peptides
There is a demand for site-selective peptide/protein conjugation chemistry that is fully reversible in a stimulus-responsive manner. The contemporary methods for site-selective protein modification enable the preparation of homogeneous protein–small molecule conjugates, which are indispensable for drug delivery and chemical biology purposes, but such chemistries are usually irreversible. In contrast, the existing reversible protein labeling techniques are generally not site-selective. Here, we report an mRNA display-enabled de novo discovery of a pair of peptides which selectively react with each other to form a conjugate that is stable under neutral conditions (pH 7.5) but rapidly dissociates into the constituents at pH 10. A Cys thiol of peptide CP1 rapidly reacts (k1 = 340 M–1·s–1) with the isothiocyanate moiety in partner ITC6 to furnish a stable dithiocarbamate (t1/2 = 6 h at pH 7.5). We show that the pH-responsive nature of the reaction (conjugate’s t1/2 = 5 min at pH 10) can be leveraged to utilize ITC6 (1) as a pull-down handle to selectively isolate CP1 from cell lysates and (2) as a temporary protecting group to protect CP1 from nonspecific Cys labeling reagents such as iodoacetamide. Altogether, the chemistry developed here complements the existing approaches and is applicable in a variety of chemical biology settings where selective reversible reactions are needed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


