NoxO1 通过与 Erbin 的相互作用调节表皮生长因子受体的信号转导。
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
NADPH 氧化酶组织者 1(NoxO1)是形成活性氧(ROS)的 Nox1 复合物的支架细胞质亚基,参与血管生成、分化和动脉粥样硬化。我们发现,过表达 NoxO1 而不同时过表达活性 Nox1 复合物的任何其他成分都会抑制 EGF 诱导的伤口闭合和信号传导,而 NoxO1 KO 则产生相反的效果。因此,我们推测 NoxO1 具有独立于 Nox1 的功能。利用 BioID 技术,我们发现 ErbB2 互作蛋白(Erbin)是 NoxO1 的新型互作伙伴。NoxO1与表皮生长因子受体以及Erbin的共定位验证了这一发现。EGF 处理中断了 NoxO1 与表皮生长因子受体的共定位。在过表达 NoxO1 的细胞中,EGF 介导的激酶激活被延迟,而敲除 NoxO1 则产生相反的效果。总之,Erbin 被鉴定为一种新型 NoxO1 相互作用蛋白。通过 NoxO1 与表皮生长因子受体的相互作用,NoxO1 干扰了表皮生长因子受体的信号转导。这项研究结果表明,NoxO1 作为一种适配蛋白,其潜在作用超出了 Nox1 介导的 ROS 形成的既定功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
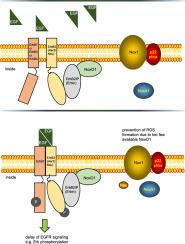
NoxO1 regulates EGFR signaling by its interaction with Erbin
NADPH oxidase organizer 1 (NoxO1) is a scaffold cytoplasmic subunit of the reactive oxygen species (ROS) forming Nox1 complex and involved in angiogenesis, differentiation, and atherosclerosis.
We found that overexpression of NoxO1 without simultaneous overexpression of any other component of the active Nox1 complex inhibited EGF-induced wound closure and signaling, while NoxO1 KO yielded the opposite effect. Accordingly, we hypothesize NoxO1 to exert Nox1 independent functions.
Using the BioID technique, we identified ErbB2 interacting protein (Erbin) as novel interaction partner of NoxO1. Colocalization of NoxO1 with EGFR, as well as with Erbin validated this finding. EGF treatment interrupted colocalization of NoxO1 and EGFR. EGF mediated kinase activation was delayed in NoxO1 overexpressing cells, while knockout of NoxO1 had the opposite effect.
In conclusion, Erbin was identified as a novel NoxO1 interacting protein. Through the subsequent interaction of NoxO1 and EGFR, NoxO1 interferes with EGF signaling. The results of this study suggest a potential role of NoxO1 as an adaptor protein with functions beyond the well-established enabling of Nox1 mediated ROS formation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


