通过乳酮醋酸酯与环烯酮的一锅转化实现扩环。合成 (±)-1-epi-Xerantholide
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
α-烷氧基酮 1 的拜尔-维利格氧化反应可生成内酯缩醛 2,在 LaCl3-2LiCl 的存在下,内酯缩醛 2 与二甲基(烷基)膦酸锂盐发生反应,在用稀碳酸钾水溶液处理后,可生成收率良好甚至极佳的环状烯酮 3。这样,五元、六元和七元内酯就转化成了五元、六元和七元环状烯酮。在从 5-甲基-2-环己烯-1-酮合成 (±)-1-epi-xerantholide 的过程中,证明了这种两步扩环法的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
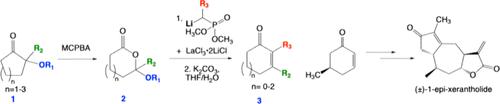
Ring Expansion via One-Pot Conversion of Lactone Acetals to Cyclic Enones. Synthesis of (±)-1-epi-Xerantholide
Baeyer–Villiger oxidation of α-alkoxy ketones 1 provides lactone acetals 2, which react with the lithium salts of dimethyl(alkyl) phosphonates in the presence of LaCl3·2LiCl to provide cyclic enones 3 in good to excellent yields after treatment with dilute aqueous potassium carbonate. Thus, five-, six-, and seven-membered lactones are converted to five-, six-, and seven-membered cyclic enones. The utility of this two-step ring expansion method is demonstrated in the synthesis of (±)-1-epi-xerantholide from 5-methyl-2-cyclohexen-1-one.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


