铜(II)介导的 2-(5-苯基异恶唑-3-基)苯胺的双重反应活性:酰胺的定向胺化和氧化 C(═O)-C 裂解,从而直接获得尿素衍生物
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报告了以 2-(5-苯基异恶唑-3-基)苯胺为指导基团,通过化学特异性裂解 C(═O)-C 键和 C(sp2)-H amination 形成脲衍生物的酰胺的双重反应性。该方法采用廉价的铜作为催化剂,以空气中的 O2 作为氧化剂,对这两种转化具有广泛的官能团耐受性。通过详细的机理研究和 DFT 计算,深入了解了脲衍生物形成的合理机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
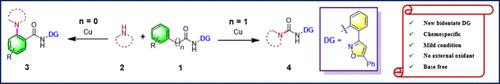
Copper(II)-Mediated Dual Reactivity of 2-(5-Phenylisoxazol-3-yl)aniline: Directed Amination and Oxidative C(═O)─C Cleavage of Amides Enabling Direct Access to Urea Derivatives
The dual reactivity of amides fused to 2-(5-phenylisoxazol-3-yl)aniline as a directing group for the formation of urea derivatives via chemospecific cleavage of the C(═O)─C bond and C(sp2)–H amination is reported here. Employing inexpensive copper as the catalyst and O2 in air as the oxidant, this protocol exhibited broad functional group tolerance for both the transformations. Detailed mechanistic studies and DFT calculations were performed to gain insights into a plausible mechanism for the formation of urea derivatives.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


