用于胆红素纳米级分析的重组融合蛋白 HUG 的标准化实验室规模生产
IF 1.6
Q2 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
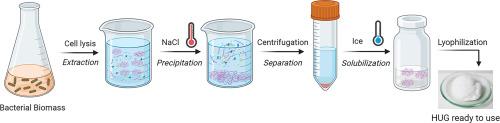
重组双功能蛋白 HELP-UnaG (HUG) 是人弹性蛋白样多肽 (HELP) 与胆红素结合荧光蛋白 UnaG 的融合产物。HUG 可用于血清、各种生物液体和提取物中胆红素的荧光检测。在此,我们介绍了一种在实验室规模上从大肠杆菌提取物中标准化生产和纯化 HUG 的详细方法。这种方法利用了 HELP 特有的热反应行为,在接近生理温度的条件下,通过重复沉淀/再溶解步骤,从复杂的大肠杆菌提取物中分离出 HUG。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Standardized lab-scale production of the recombinant fusion protein HUG for the nanoscale analysis of bilirubin
The recombinant bifunctional protein HELP-UnaG (HUG) is a fusion product of the Human Elastin-like Polypeptide (HELP) with the bilirubin-binding fluorescent protein UnaG. HUG is used for the fluorometric detection of bilirubin in serum and a variety of biological fluids and extracts. Here we describe a detailed method for the standardized production and purification of HUG from E. coli extracts on a laboratory scale. This method takes advantage of the HELP-specific thermoreactive behavior that enables the separation of HUG from complex E. coli extracts by repeated precipitation/re-dissolution steps at near physiological temperature.
- •The method is based on the inverse thermal transition process.
- •The “green” method is affordable for basic laboratories and can be easily transferred to new users.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

MethodsX
Health Professions-Medical Laboratory Technology
CiteScore
3.60
自引率
5.30%
发文量
314
审稿时长
7 weeks
期刊介绍:
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


