聚合物纳米盘支持人工跨膜细胞色素的功能提取
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在研究原生脂质环境中的整体膜蛋白时,聚合物纳米盘是一种极具吸引力的表面活性剂替代品。在这里,我们研究了如何利用这种聚合物来分离一种通过计算重新设计的膜细胞色素 CytbX。我们的研究表明,被称为 CyclAPols 的嵌段共聚物能直接从生物膜中有效地提取 CytbX,并能与这种人工蛋白的下游纯化和生物物理特性分析相兼容。CyclAPol溶解后的CytbX具有良好的折叠性和高度的稳健性,其特性与在洗涤剂胶束中观察到的相同蛋白质的特性基本相同。然而,在胶束中,扩散黄素蛋白向 CytbX 的电子传递速度远远快于纳米盘系统。我们的研究结果证实,聚合物纳米盘将成为研究和应用新膜蛋白的有用工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
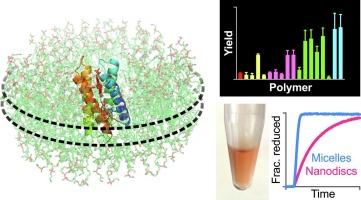
Polymer nanodiscs support the functional extraction of an artificial transmembrane cytochrome
Polymer nanodiscs are an attractive alternative to surfactants for studying integral membrane proteins within their native lipid environment. Here, we investigate the use of such polymers to isolate a computationally-designed de novo membrane cytochrome named CytbX. We show that the block copolymers known as CyclAPols can efficiently extract CytbX directly from biomembranes and are compatible with the downstream purification and biophysical characterisation of this artificial protein. CyclAPol-solubilised CytbX is well-folded and highly robust with properties that are essentially identical to those observed for the same protein in a detergent micelle. However, electron transfer to CytbX from a diffusive flavoprotein is substantially faster in micelles than in the nanodisc system. Our results confirm that polymer nanodiscs will be a useful tool for the ongoing study and application of de novo membrane proteins.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochimica et biophysica acta. Biomembranes
生物-生化与分子生物学
CiteScore
8.20
自引率
5.90%
发文量
175
审稿时长
2.3 months
期刊介绍:
BBA Biomembranes has its main focus on membrane structure, function and biomolecular organization, membrane proteins, receptors, channels and anchors, fluidity and composition, model membranes and liposomes, membrane surface studies and ligand interactions, transport studies, and membrane dynamics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


