嵌入氮、硫掺杂碳/MXene 的磷化钴上的电子转移机制引发的优异双酚 A 去除性能
IF 9.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
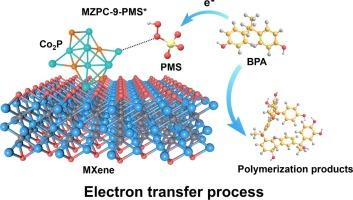
在以过硫酸盐为基础的高级氧化工艺中,以电子转移过程(ETP)为主的非自由基途径在去除有机污染物方面受到了广泛关注。合理设计具有优化组成和结构优点的新型催化剂并进一步阐明其增强去除机理具有重要意义。在这项工作中,我们成功合成了一种氮硫共掺杂碳,在 MXene 纳米片(MZPC)两面包覆磷化钴(Co2P),用于降解有机废水中的双酚 A(BPA)。结果表明,使用 0.1 g L-1 的过一硫酸盐 (PMS) 和 0.05 g L-1 的优化催化剂 (MZPC-9),双酚 A 在短短 5 分钟内降解了 98.2%,表现出极佳的伪一阶动力学速率常数 (k = 1.485 min-1)。均匀分散在 MXene 两侧的 Co2P 纳米颗粒(约 9.4 nm,使用舍勒方程计算)与 PMS 的结合亲和力增强,形成了具有强大氧化能力的 MZPC-9-PMS* 可转移复合物。由此产生的 MZPC-9-PMS* 复合物诱导了双酚 A 的聚合反应,并实现了 81% 的总有机碳 (TOC) 去除率。这项研究为设计金属活性中心以增强 ETP 主导的非自由基污染物降解途径提供了一个新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Excellent bisphenol A removal performance triggered by electron-transfer regime on cobalt phosphide embedded in nitrogen, sulfur-doped carbon/MXene
The non-radical pathway dominated by the electron transfer process (ETP) has gained considerable attention for the removal of organic contaminants in persulfate-based advanced oxidation processes. Rationally designing new catalysts with optimized composition and structural merits and further elucidating the enhanced removal mechanism are of great importance. In this work, we successfully synthesized a nitrogen-sulfur co-doped carbon encapsulated cobalt phosphide (Co2P) on both sides of MXene nanosheets (MZPC) to degrade bisphenol A (BPA) from organic wastewater. The results indicated that BPA was degraded by 98.2 % in a mere 5 min using 0.1 g L-1 of peroxymonosulfate (PMS) and 0.05 g L-1 of the optimized catalyst (MZPC-9), exhibiting an excellent pseudo-first-order kinetics rate constant (k = 1.485 min−1). Uniformly dispersed Co2P nanoparticles (approximately 9.4 nm, calculated using the Scherrer equation) on both sides of MXene exhibited enhanced binding affinity with PMS, forming the MZPC-9-PMS* metastable complexes with potent oxidative capability. The resultant MZPC-9-PMS* complexes induced the polymerization reaction of BPA and achieved 81 % total organic carbon (TOC) removal. This study offers a novel perspective on the design of metal active centers to enhance the ETP-dominated non-radical pathway for pollutant degradation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


