3,5-双((2-羟基亚苄基)氨基)-N-(2-羟基苯基)苯甲酰胺和 Zn(II) 复合物的合成与表征:色度、荧光和 DPPH 自由基清除行为的研究
IF 2.7
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
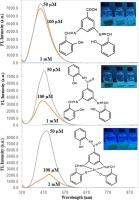
在偶联剂 N,N′-二环己基碳二亚胺的存在下,通过希夫碱 1a 与 2-羟基苯胺的反应,得到了新型氨基希夫碱 3,5-双((2-羟基苯亚甲基)氨基)-N-(2-羟基苯基)苯甲酰胺 (2a)。作为酰胺合成起始化合物的希夫碱(1a)是由 3,5-二氨基苯甲酸与水杨醛以 1:2 的摩尔比缩合而成。配体 2a 与硝酸锌(II)发生络合反应,新合成了具有 1:1 [M:L] 配比的四面体锌(II)络合物(3a)。在纯二甲基亚砜中,1a 和 2a 在 λem = 456 和 472 nm 处显示出宽广的发射带,并发出蓝色荧光,而 3a 在 λem = 485 nm 处发出强烈的绿蓝色荧光(λexc = 365 nm)。与其他物质相比,1a 的荧光效率较低。在二甲基亚砜水溶液(v/v,1:1)中的λem 与纯溶液相比有 8-33 nm 的红移。在酸性 DMSO 介质中,λem 的红移为 1-15 nm,发射效率非常低。结果表明,1a-3a 在纯水和酸性 DMSO(含 50% 水分)体系中的聚集导致了淬灭特性。在碱性 DMSO 介质中,化合物的荧光显得非常明亮。还利用紫外可见光谱对所有化合物的抗氧化活性进行了筛选。结果发现,这些化合物在 DMSO 中不是有效的 DPPH 自由基清除剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and characterization of 3,5-bis((2-hydroxybenzylidene)amino)-N-(2-hydroxyphenyl)benzamide and Zn(II) complex: Investigation of chromic, fluorescence and DPPH radical scavenging behaviours
Novel amido-Schiff base 3,5-bis((2-hydroxybenzylidene)amino)-N-(2-hydroxyphenyl)benzamide (2a) has been obtained by the reaction of Schiff base 1a with 2-hydroxyaniline in the presence of coupling agent N,N′-dicyclohexylcarbodiimide. Schiff base (1a) which serves as the starting compound in amide synthesis was produced from the condensation of 3,5-diaminobenzoic acid with salicylaldehyde in a 1:2 mol ratio. Tetrahedral geometry with 1:1 [M:L] stoichiometry Zn(II) complex (3a) was newly synthesized by the complexation reaction of ligand 2a with zinc(II) nitrate. 1a and 2a displayed a broad emission band at λem = 456 and 472 nm with a blue fluorescent in pure DMSO, while 3a emitted a strong greenish-blue fluorescent with the λem = 485 nm (λexc = 365 nm). The fluorescence efficiency of 1a was poor compared to the others. The λem in aqueous DMSO solution (v/v, 1:1) had ∼8–33 nm red-shift with respect to that of pure solution. The λem gave ∼1–15 nm red-shift with very low emission efficiency in acidic DMSO media. The results suggested that the aggregation caused quenching properties of 1a–3a in pure, aqueous, and acidic DMSO system with a 50 % water fraction. The fluorescence of the compounds appeared to be visibly very bright in basic DMSO media. All the compounds were also screened for antioxidant activity using UV–Vis spectroscopy. They were found to be not effective DPPH radical scavengers in DMSO.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


