口服葡萄糖刺激胰岛素释放的基因结构为 2 型糖尿病病因提供了生物学启示
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
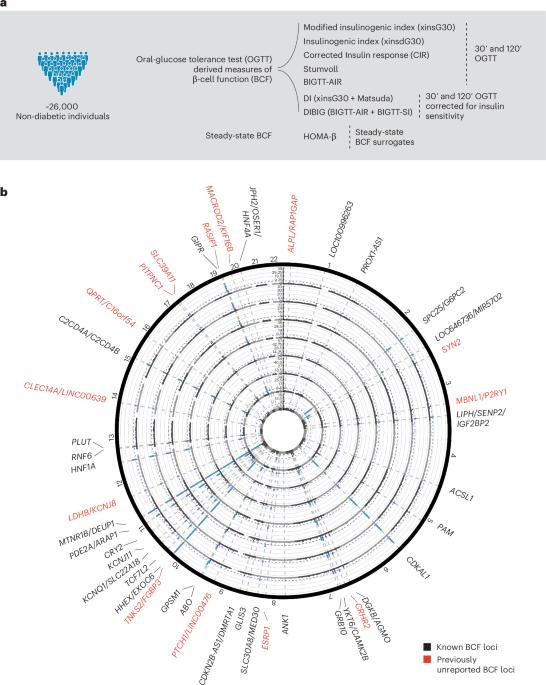
β细胞功能(BCF)的遗传学为 2 型糖尿病(T2D)的病因学提供了宝贵的见解1,2。以往的研究通过候选基因研究3,4,5,6,7、空腹 BCF 的大规模全基因组关联研究(GWAS)8,9 或有关 T2D 风险变异的功能性胰岛研究10,11,12,13,14,扩大了 BCF 遗传关联的目录。然而,以口服葡萄糖耐量试验(OGTT)数据得出的 BCF 特质为重点的全基因组关联研究(GWAS)样本量有限15,16,而且往往忽略了相关特质捕捉胰岛素分泌 β 细胞不同遗传特征的潜力17,18。我们认为,研究多个 BCF 估计值的遗传基础可以更广泛地了解 β 细胞生理学。在这里,我们汇总了来自约 26,000 名欧洲后裔的八个基于 OGTT 的 BCF 特质的 GWAS 数据,在 44 个位点上发现了 55 个独立的遗传关联。通过研究 BCF 遗传信号对相关表型的影响,我们发现了 BCF 遗传调控可能影响 T2D 风险的多种疾病机制。将 BCF-GWAS 数据与胰岛转录组和表观基因组数据集整合,发现了 92 个候选效应基因。β细胞模型中的基因沉默突显出 ACSL1 和 FAM46C 是胰岛素分泌的关键调控因子。总之,我们的研究结果深入揭示了胰岛素释放的生物学特性以及将 BCF 与 T2D 风险联系起来的分子过程,揭示了 T2D 病理生理学的异质性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Genetic architecture of oral glucose-stimulated insulin release provides biological insights into type 2 diabetes aetiology
The genetics of β-cell function (BCF) offer valuable insights into the aetiology of type 2 diabetes (T2D)1,2. Previous studies have expanded the catalogue of BCF genetic associations through candidate gene studies3–7, large-scale genome-wide association studies (GWAS) of fasting BCF8,9 or functional islet studies on T2D risk variants10–14. Nonetheless, GWAS focused on BCF traits derived from oral glucose tolerance test (OGTT) data have been limited in sample size15,16 and have often overlooked the potential for related traits to capture distinct genetic features of insulin-producing β-cells17,18. We reasoned that investigating the genetic basis of multiple BCF estimates could provide a broader understanding of β-cell physiology. Here, we aggregate GWAS data of eight OGTT-based BCF traits from ~26,000 individuals of European descent, identifying 55 independent genetic associations at 44 loci. By examining the effects of BCF genetic signals on related phenotypes, we uncover diverse disease mechanisms whereby genetic regulation of BCF may influence T2D risk. Integrating BCF-GWAS data with pancreatic islet transcriptomic and epigenomic datasets reveals 92 candidate effector genes. Gene silencing in β-cell models highlights ACSL1 and FAM46C as key regulators of insulin secretion. Overall, our findings yield insights into the biology of insulin release and the molecular processes linking BCF to T2D risk, shedding light on the heterogeneity of T2D pathophysiology. In a genome-wide association study for traits related to pancreatic beta-cell function in 26,000 individuals, 55 independent associations mapping to 44 genetic loci are identified.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


