福里斯特肽通过痛觉神经元中的 IGF1R 信号驱动小鼠的神经性疼痛
IF 15.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
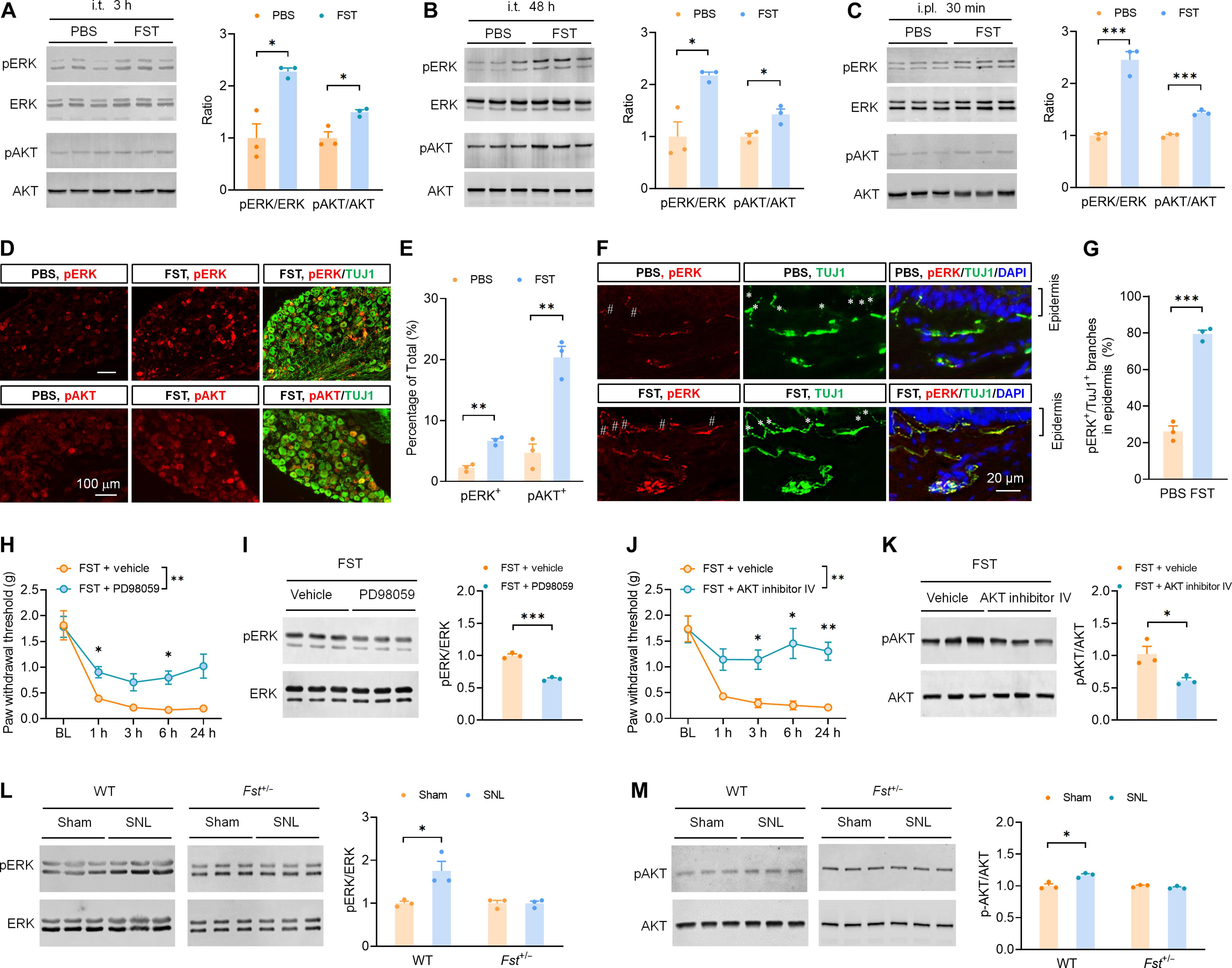
神经性疼痛是一种使人衰弱的慢性疾病,缺乏有效的治疗方法。细胞因子和趋化因子介导的神经炎症在其发病机制中的作用已得到充分证实。已知 Follistatin(FST)是一种分泌蛋白,可拮抗转化生长因子-β(TGF-β)超家族细胞因子的生物活性。FST 在神经病理性疼痛中的参与及其潜在机制在很大程度上仍不为人所知。在此,我们报告了小鼠脊神经结扎(SNL)后 A 纤维感觉神经元中 FST 的上调。抑制或删除 FST 可减轻神经病理性疼痛,并降低 SNL 诱导的痛觉神经元过度兴奋性。相反,鞘内或跖内注射重组 FST 或在背根神经节(DRG)神经元中过表达 FST 会诱导痛觉过敏。此外,外源性 FST 还能提高痛觉神经元的兴奋性。生物层干涉测量法(BLI)测定和共沉淀免疫法(co-immunoprecipitation,co-IP)证明了FST与胰岛素样生长因子-1受体(IGF1R)的直接结合,抑制IGF1R可减少FST诱导的细胞外信号调节激酶(ERK)和蛋白激酶B(AKT)的激活以及神经元的过度兴奋。进一步的 coIP 分析表明,FST 的 N 端结构域与 IGF1R 的亲和力最高,用一种来自 FST 的多肽阻断这种相互作用可减轻 Nav1.7 介导的神经元过度兴奋性和 SNL 后的神经病理性疼痛。此外,FST 还能通过 IGF1R 增强人 DRG 神经元的兴奋性。总之,我们的研究结果表明,从A纤维神经元释放的FST可通过与IGF1R结合增强Nav1.7介导的痛觉神经元过度兴奋性,使其成为治疗神经性疼痛的潜在靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Follistatin drives neuropathic pain in mice through IGF1R signaling in nociceptive neurons
Neuropathic pain is a debilitating chronic condition that lacks effective treatment. The role of cytokine- and chemokine-mediated neuroinflammation in its pathogenesis has been well documented. Follistatin (FST) is a secreted protein known to antagonize the biological activity of cytokines in the transforming growth factor–β (TGF-β) superfamily. The involvement of FST in neuropathic pain and the underlying mechanism remain largely unknown. Here, we report that FST was up-regulated in A-fiber sensory neurons after spinal nerve ligation (SNL) in mice. Inhibition or deletion of FST alleviated neuropathic pain and reduced the nociceptive neuron hyperexcitability induced by SNL. Conversely, intrathecal or intraplantar injection of recombinant FST, or overexpression of FST in the dorsal root ganglion (DRG) neurons, induced pain hypersensitivity. Furthermore, exogenous FST increased neuronal excitability in nociceptive neurons. The biolayer interferometry (BLI) assay and coimmunoprecipitation (co-IP) demonstrated direct binding of FST to the insulin-like growth factor–1 receptor (IGF1R), and IGF1R inhibition reduced FST-induced activation of extracellular signal–regulated kinase (ERK) and protein kinase B (AKT), as well as neuronal hyperexcitability. Further co-IP analysis revealed that the N-terminal domain of FST exhibits the highest affinity for IGF1R, and blocking this interaction with a peptide derived from FST attenuated Nav1.7-mediated neuronal hyperexcitability and neuropathic pain after SNL. In addition, FST enhanced neuronal excitability in human DRG neurons through IGF1R. Collectively, our findings suggest that FST, released from A-fiber neurons, enhances Nav1.7-mediated hyperexcitability of nociceptive neurons by binding to IGF1R, making it a potential target for neuropathic pain treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Translational Medicine
CELL BIOLOGY-MEDICINE, RESEARCH & EXPERIMENTAL
CiteScore
26.70
自引率
1.20%
发文量
309
审稿时长
1.7 months
期刊介绍:
Science Translational Medicine is an online journal that focuses on publishing research at the intersection of science, engineering, and medicine. The goal of the journal is to promote human health by providing a platform for researchers from various disciplines to communicate their latest advancements in biomedical, translational, and clinical research.
The journal aims to address the slow translation of scientific knowledge into effective treatments and health measures. It publishes articles that fill the knowledge gaps between preclinical research and medical applications, with a focus on accelerating the translation of knowledge into new ways of preventing, diagnosing, and treating human diseases.
The scope of Science Translational Medicine includes various areas such as cardiovascular disease, immunology/vaccines, metabolism/diabetes/obesity, neuroscience/neurology/psychiatry, cancer, infectious diseases, policy, behavior, bioengineering, chemical genomics/drug discovery, imaging, applied physical sciences, medical nanotechnology, drug delivery, biomarkers, gene therapy/regenerative medicine, toxicology and pharmacokinetics, data mining, cell culture, animal and human studies, medical informatics, and other interdisciplinary approaches to medicine.
The target audience of the journal includes researchers and management in academia, government, and the biotechnology and pharmaceutical industries. It is also relevant to physician scientists, regulators, policy makers, investors, business developers, and funding agencies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


