通过支撑诱导策略调节 Ni3N@Mo2C 上的 Ru 物种,增强氢氧化作用
IF 2.8
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
使用氢气作为中间体来转换和储存电化学能量一直是人们非常感兴趣和关注的话题。遗憾的是,缓慢的碱性氢氧化反应(HOR)阻碍了氢氧燃料电池的进一步发展。由于钌(Ru)与铂(Pt)具有相似的氢结合能(HBE),最近有人将其作为氢氧化反应中铂(Pt)催化剂的替代品进行了研究。该催化剂的交换电流密度和动力学电流密度分别为 3.05 和 4.76 mA cmdisk-2,分别是商用 Pt/C 的 2 倍和 1.4 倍。研究结果表明,Ni3N@Mo2C 支持降低了 Ru 位点上的氢结合能。这改善了 HOR 的 Volmer 步骤,提高了催化活性。因此,这项研究为设计高效氢能转化的 HOR 催化剂提供了一些指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
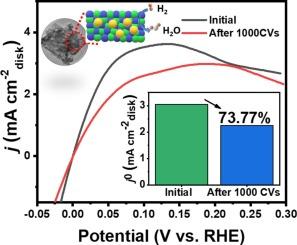
Enhancing hydrogen oxidation by Modulating Ru species on Ni3N@Mo2C through a Support-Induced Strategy
The use of hydrogen as an intermediator to convert and store electrochemical energy has been a subject of significant interest and focus. Unfortunately, the slow alkaline hydrogen oxidation reaction (HOR) is a barrier to further development of hydrogen–oxygen fuel cells. Ruthenium (Ru) has recently been investigated as a possible replacement for platinum (Pt) catalyst in the HOR because of the similar hydrogen binding energy (HBE) to Pt. Herein, Ru species was loaded on Ni3N@Mo2C support, which was used as an electrocatalyst for HOR. The catalyst presents an exchange current density and kinetic current densities of 3.05 and 4.76 mA cmdisk−2 that are 2 and 1.4 times greater than that of commercial Pt/C, respectively. The findings indicate that the Ni3N@Mo2C support reduces the hydrogen binding energy on Ru sites. This improves the Volmer step for HOR and increases the catalytic activity. This study thus provides some guidance in the designing of HOR catalysts for efficient hydrogen energy conversion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics Letters
化学-物理:原子、分子和化学物理
CiteScore
5.70
自引率
3.60%
发文量
798
审稿时长
33 days
期刊介绍:
Chemical Physics Letters has an open access mirror journal, Chemical Physics Letters: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Chemical Physics Letters publishes brief reports on molecules, interfaces, condensed phases, nanomaterials and nanostructures, polymers, biomolecular systems, and energy conversion and storage.
Criteria for publication are quality, urgency and impact. Further, experimental results reported in the journal have direct relevance for theory, and theoretical developments or non-routine computations relate directly to experiment. Manuscripts must satisfy these criteria and should not be minor extensions of previous work.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


