微调铱(III)配合物的侧链长度以增强癌症治疗的光物理特性
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
环金属化铱(III)复合物已成为癌症治疗的多功能候选物质,可提供综合诊断成像和强效单线态氧(1O2)生成,用于光动力疗法(PDT)。然而,主要由于分子内运动引起的激发能量耗散,它们的应用一直受到光致发光不足的限制。在本研究中,我们通过设计和合成五种新型铱(III)配合物来解决这些局限性:IrC2、IrC4、IrC6、IrC8 和 IrC12。我们的方法采用了细致的侧链延长策略来调节侧链长度,从而减少了分子内运动,并显著增强了聚集态的单光子和三光子发射以及 1O2 生成。详细的光物理研究以及对晶体学的深入了解表明,侧链的拉长大大增强了这些特性。在合成的复合物中,IrC8 脱颖而出,成为在细胞和三维肿瘤球体模型中进行图像引导光动力疗法的理想候选物质。这项研究开创性地在铱(III)复合物中通过新型侧链延伸策略同时增强了双光子发射和光动力疗法的疗效,为其在临床治疗学中的转化应用铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
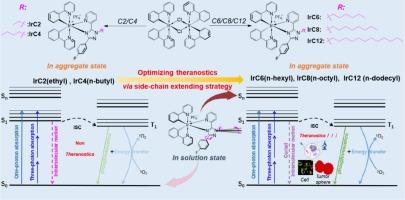
Fine-tuning the side-chain length of iridium(III) complexes for enhanced Photophysical properties in Cancer Theranostics
Cyclometalated iridium(III) complexes have emerged as versatile candidates for cancer theranostics, offering integrated diagnostic imaging and potent singlet oxygen (1O2) generation for photodynamic therapy (PDT). However, their application has been limited by subdued photoluminescence, primarily due to intramolecular motion-induced excited energy dissipation. In this study, we address these limitations through the design and synthesis of five novel iridium(III) complexes: IrC2, IrC4, IrC6, IrC8, and IrC12. Our approach employs meticulous side-chain extending strategy to modulate side-chain length, thereby reducing intramolecular motion and significantly enhancing both one- and three-photon emissions and 1O2 production in the aggregated state. Detailed photophysical investigations, supported by crystallographic insights, reveal that side-chain elongation substantially amplifies these properties. Among the synthesized complexes, IrC8 stands out as a superior candidate for image-guided photodynamic therapy in cellular and 3D tumor spheroid models. This investigation pioneers the simultaneous enhancement of dual-photon emissions and PDT efficacy through a novel side-chain extension strategy in iridium(III) complexes, paving the way for their translational application in clinical theranostics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


