从纤维粘连蛋白和β1整合素转基因小鼠身上了解转移性骨髓龛位
IF 7.7
2区 医学
Q1 Biochemistry, Genetics and Molecular Biology
引用次数: 0
摘要
肿瘤细胞可从原发癌症转移到其他器官(包括骨髓)内的龛位,从而形成转移灶,在骨髓中,肿瘤细胞可利用造血干细胞龛位。转移前壁龛和造血干细胞壁龛的确切组成以及它们之间的重叠程度仍难以确定。由于细胞外基质蛋白纤连蛋白在转移前肺微环境中表达,我们评估了其缺失的影响,以及其主要受体亚基β1整合素缺失对乳腺癌骨转移和造血过程中各种骨髓细胞类型的影响。我们利用八种转基因小鼠模型证实,表达 osterix 的骨髓细胞产生的纤维粘连蛋白或 β1 整合素的表达(在 vav、mx 或瘦素受体表达细胞上)会影响骨髓中 MDA-MB-231 乳腺癌细胞的数量。此外,我们还发现了可调节通过血管壁转移的基质亚群。决定肿瘤是否恶化的不是肿瘤细胞的数量,而是微环境的变化。总之,骨髓中的转移前壁龛和造血壁龛之间存在部分重叠。总之,骨髓中的转移前壁龛和造血壁龛之间存在部分重叠。此外,我们还描述了一个由前成骨细胞分泌的纤维粘连蛋白开始的级联过程,它有可能作用于特定基质细胞亚群的β1整合素,从而抑制新的乳腺癌病灶在骨髓中的形成。因此,这项研究揭示了影响肿瘤行为和影响造血的各种基质细胞亚群的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
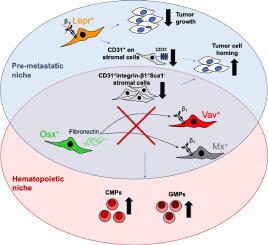
Insights into the metastatic bone marrow niche gained from fibronectin and β1 integrin transgenic mice
Tumor cells can migrate from a primary cancer and form metastases by localizing to niches within other organs including the bone marrow, where tumor cells may exploit the hematopoietic stem cell niche. The precise composition of the premetastatic and the hematopoietic niches and the degree of overlap between them remain elusive. Because the extracellular matrix protein fibronectin is expressed in the pre-metastatic lung microenvironment, we evaluated the implications of its loss, as well as those of loss of its primary receptor subunit, β1 integrin, in various bone marrow cell types both in breast cancer bone metastasis and hematopoiesis.
Using eight transgenic mouse models, we established that fibronectin production by osterix-expressing marrow cells, or β1 integrin expression (on vav, mx, or leptin receptor expressing cells), affects MDA-MB-231 breast cancer cell numbers in the bone marrow. Additionally, we identified stromal subpopulations that modulate transmigration through blood vessel walls. Not the number of tumor cells, but rather the changes in the microenvironment dictated whether the tumor progresses. Furthermore, hematopoiesis, particularly myelopoiesis, was affected in some of the models showing changes in tumor homing.
In conclusion, there is partial overlap between the pre-metastatic and the hematopoietic niches in the bone marrow. Moreover, we have delineated a cascade starting with fibronectin secreted by pre-osteoblastic cells, which potentially acts on β1 integrin in specific stromal cell subsets, thereby inhibiting the formation of new breast cancer lesions in the bone marrow. This work therefore sheds light on the role of various stromal cell subpopulations that influence tumor behavior and affect hematopoiesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neoplasia
医学-肿瘤学
CiteScore
9.20
自引率
2.10%
发文量
82
审稿时长
26 days
期刊介绍:
Neoplasia publishes the results of novel investigations in all areas of oncology research. The title Neoplasia was chosen to convey the journal’s breadth, which encompasses the traditional disciplines of cancer research as well as emerging fields and interdisciplinary investigations. Neoplasia is interested in studies describing new molecular and genetic findings relating to the neoplastic phenotype and in laboratory and clinical studies demonstrating creative applications of advances in the basic sciences to risk assessment, prognostic indications, detection, diagnosis, and treatment. In addition to regular Research Reports, Neoplasia also publishes Reviews and Meeting Reports. Neoplasia is committed to ensuring a thorough, fair, and rapid review and publication schedule to further its mission of serving both the scientific and clinical communities by disseminating important data and ideas in cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


