在 NLRP3 炎症小体激活过程中,MEK1/2 可促进 ROS 生成,并独立于 ERK1/2 对 NLRP3 进行去泛素化处理
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
炎症小体是一种细胞膜超分子复合物,在先天性免疫反应中发挥着关键作用。NLR 家族含吡啶域 3(NLRP3)炎性体的过度激活会导致多种疾病。翻译后修饰(PTM)是炎性小体的重要调节因子,尤其是在激活阶段。在这里,我们发现MEK1/2激酶的活性在体外和体内激活NLRP3炎症小体的过程中都是不可或缺的。抑制 MEK1/2 可清除活性氧(ROS)并使 NLRP3 泛素化,从而进一步阻断 NLRP3 炎症小体的激活。这些作用与ERK1/2无关,而ERK1/2是MEK1/2的经典下游。这些研究提出了MEK1/2通过NLRP3炎性体的非转录调控来调节炎症的机制,可能有助于更好地理解临床使用的MEK抑制剂的作用和副作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
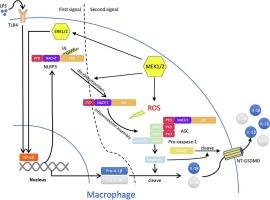
MEK1/2 promote ROS production and deubiquitinate NLRP3 independent of ERK1/2 during NLRP3 inflammasome activation
Inflammasomes are cytosolic supramolecular complexes that play a key role in the innate immune response. Overactivation of NLR family pyrin domain containing 3 (NLRP3) inflammasome leads to multiple diseases. Post-translational modifications (PTMs) are essential modulators of inflammasomes especially in activation phase. Here we found that MEK1/2 kinase activity was indispensable in NLRP3 inflammasome activation both in vitro and in vivo. Inhibition of MEK1/2 resulted in reactive oxygen species (ROS) scavenging and ubiquitination of NLRP3, which further blocked NLRP3 inflammasome activation. These effects were independent of ERK1/2, which were classic downstream of MEK1/2. These investigations proposed a mechanism that MEK1/2 regulated inflammation via non-transcriptional regulation of NLRP3 inflammasome and might help better understanding the effects and side-effects of MEK inhibitors in clinical use.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


