二氧化碳在 PuO2 和 α-Pu2O3 表面的反应机理
IF 2.8
2区 工程技术
Q3 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
在 DFT+U-D3 方案中,我们系统地研究了二氧化碳在氧化钚表面反应的主要步骤,包括二氧化碳吸附、解离和二氧化碳释放。在完整的氧化钚表面,二氧化碳的化学吸附需要激活,而解离则不太可能发生。有趣的是,当氧化钚表面被*H或解离出的H2O产生的*OH覆盖时,这两个步骤都能得到明显的促进,这与实验结果完全一致,即水分能促进二氧化碳在氧化钚表面的吸附。具体来说,*H 或*OH 诱导的极子与 CO2 发生强耦合,首先激活 CO2 的无阻化学吸附,然后通过削弱 CO 键促进 CO2 的解离。此外,我们还发现,钚在其氧化物中的氧化态决定了 CO 释放步骤的趋势,在 PuO2 和 α-Pu2O3 表面,CO 释放步骤分别为内热和放热。因此,我们预测二氧化碳在钚氧化物表面的反应并非不可能,尤其是当钚氧化物在长期储存过程中还原为α-钚氧化物时。这项工作揭示了二氧化碳在钚氧化物上的反应机理和可能的反应途径,为了解锕系元素氧化物的表面反应迈出了重要一步。本文章由计算机程序翻译,如有差异,请以英文原文为准。
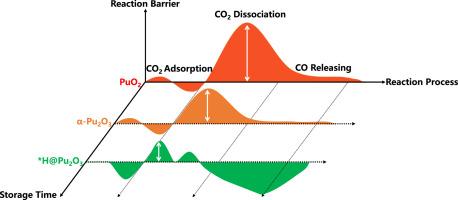
The reaction mechanism of CO2 on PuO2 and α-Pu2O3 surfaces
Within DFT+U-D3 scheme, we systematically investigate the major steps of CO2 reaction on Pu-oxide surfaces, including CO2 adsorption, dissociation and CO releasing. On intact Pu-oxide surfaces, the chemical adsorption of CO2 needs to be activated, while the dissociation is unlikely to happen. Interestingly, both steps can be notably promoted when Pu-oxide surfaces are covered with *H or *OH from dissociated H2O, which is fully consistent with the experimental findings that moisture can boosting CO2 adsorption on PuO2 surface. Specifically, the polarons induced by *H or *OH show strong couplings with CO2, which first activate the barrier-less chemisorption of CO2 and then promote the dissociation of CO2 by weaken the C![]() O bond. Furthermore, we find that the oxidation state of Pu in its oxides determines the tendency of CO releasing step, which is endothermic and exothermic on PuO2 and α-Pu2O3 surfaces, respectively. Thus, we predict the reaction of CO2 on Pu-oxide surface is not impossible, especially when PuO2 is reducing to α-Pu2O3 during the long-term storage. This work reveals the reaction mechanism and possible reactive pathway of CO2 on Pu-oxides, which provides a significant step forward in the understanding of the surface reactions of actinide oxides.
O bond. Furthermore, we find that the oxidation state of Pu in its oxides determines the tendency of CO releasing step, which is endothermic and exothermic on PuO2 and α-Pu2O3 surfaces, respectively. Thus, we predict the reaction of CO2 on Pu-oxide surface is not impossible, especially when PuO2 is reducing to α-Pu2O3 during the long-term storage. This work reveals the reaction mechanism and possible reactive pathway of CO2 on Pu-oxides, which provides a significant step forward in the understanding of the surface reactions of actinide oxides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Nuclear Materials
工程技术-材料科学:综合
CiteScore
5.70
自引率
25.80%
发文量
601
审稿时长
63 days
期刊介绍:
The Journal of Nuclear Materials publishes high quality papers in materials research for nuclear applications, primarily fission reactors, fusion reactors, and similar environments including radiation areas of charged particle accelerators. Both original research and critical review papers covering experimental, theoretical, and computational aspects of either fundamental or applied nature are welcome.
The breadth of the field is such that a wide range of processes and properties in the field of materials science and engineering is of interest to the readership, spanning atom-scale processes, microstructures, thermodynamics, mechanical properties, physical properties, and corrosion, for example.
Topics covered by JNM
Fission reactor materials, including fuels, cladding, core structures, pressure vessels, coolant interactions with materials, moderator and control components, fission product behavior.
Materials aspects of the entire fuel cycle.
Materials aspects of the actinides and their compounds.
Performance of nuclear waste materials; materials aspects of the immobilization of wastes.
Fusion reactor materials, including first walls, blankets, insulators and magnets.
Neutron and charged particle radiation effects in materials, including defects, transmutations, microstructures, phase changes and macroscopic properties.
Interaction of plasmas, ion beams, electron beams and electromagnetic radiation with materials relevant to nuclear systems.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


