双功能路易斯碱催化吡唑啉酮衍生的 MBH 碳酸酯与芳烃的(3 + 2)环加成反应
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
芳香族化合物与烯丙基醯胺的 (3 + 2) 环加成反应仍然是一项艰巨的挑战,因为这两种中间体都具有高活性,并且容易自发淬灭。在此,我们报告了吡唑啉酮 MBH 碳酸酯与芳香族化合物的 (3 + 2) 环加成反应,从而实现了多种茚并螺吡唑啉酮的高效合成。其关键在于采用了一种新型双功能路易斯碱催化剂来促进原位生成的烯丙基吡啶鎓酰化物与芳香烃的环加成反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
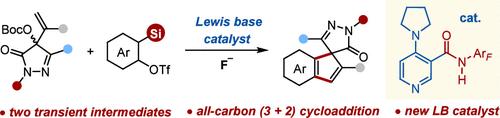
Bifunctional Lewis Base-Catalyzed (3 + 2) Cycloadditions of Pyrazolone-Derived MBH Carbonates with Arynes
The (3 + 2) cycloaddition of arynes with allylic ylides remains a formidable challenge because both intermediates are highly reactive and prone to spontaneous quenching. Here, we report a (3 + 2) cycloaddition of pyrazolone MBH carbonates with arynes, enabling the efficient synthesis of diverse indene-fused spiropyrazolones. The key is employing a new bifunctional Lewis base catalyst to facilitate the cycloaddition of in situ generated allylic pyridinium ylides with arynes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


