I 型和 II 型杂交聚酮合成酶产生不同的芳香族聚酮化合物
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
细菌芳香族多酮是由细菌 II 型多酮合成酶(PKSs)合成的具有多个芳香环的化合物,其中一些已被开发成临床药物。由 I 型和 II 型 PKSs 混合合成的含芳香族多酮化合物极为罕见。在此,我们报告了通过广泛的生物信息学分析发现的同时编码模块化 I 型和 II 型 PKSs 以及 KAS III 的基因簇,并由此确定了杂交多酮--螺环素 A 的特征。螺环素 A 的结构在所有芳香族多酮化合物中是罕见的,它具有一个独特的起始单元和四个螺环,并形成一个二聚体。生物合成研究表明,该分子的起始单元是由 I 型 PKS 与两个反式酮还原酶(KR)和烯酰还原酶(ER)合作合成的。然后,它被 KAS III 转移到 II 型 PKS 系统,后者合成三环芳香族多酮骨架。随后,螺环的形成和二聚化由基因簇中编码的四种氧化还原酶完成。总之,螺环素 A 的发现为鉴定新型芳香族多酮化合物提供了一种新方法,并为这些混合多酮化合物的生物工程提供了潜在的酶工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
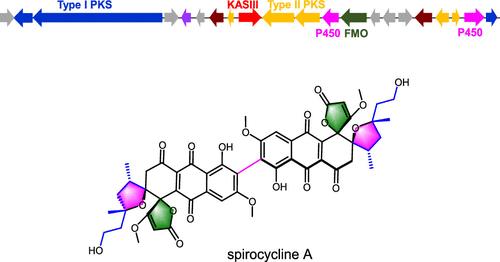
Hybrid Type I and II Polyketide Synthases Yield Distinct Aromatic Polyketides
Bacterial aromatic polyketides are compounds with multiple aromatic rings synthesized by bacterial type II polyketide synthases (PKSs), some of which have been developed into clinical drugs. Compounds containing aromatic polyketides synthesized by hybrid type I and type II PKSs are extremely rare. Here, we report the discovery of a gene cluster encoding both modular type I and type II PKSs as well as KAS III through extensive bioinformatics analysis, leading to the characterization of the hybrid polyketide, spirocycline A. The structure of spirocycline A is rare among all aromatic polyketides, featuring a unique starter unit and four spirocycles and forming a dimer. Biosynthetic studies indicate that the starter unit of this molecule is synthesized by type I PKS in collaboration with two trans-acting ketoreductase (KR) and enoylreductase (ER). It is then transferred by KAS III to the type II PKS system, which synthesizes the tricyclic aromatic polyketide backbone. The subsequent formation of the spirocycle and dimerization are carried out by four redox enzymes encoded in the gene cluster. Overall, the discovery of spirocycline A provides a new approach for identifying novel aromatic polyketides and offers potential enzymatic tools for the bioengineering of these hybrid polyketides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


