受挫路易斯对与水氧化剂共同促进氢硅烷向硅烷醇的有机催化转化
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
硅烷醇因其独特的性质而备受关注,但利用 H2O 作为绿色氧化剂对氢硅烷进行有机催化氧化制备硅烷醇仍然具有挑战性。在这里,我们利用受挫路易斯对(FLP)成功地抑制了不需要的硅氧烷的形成,并在 H2O 存在下以高产率甚至极高产率制备出了硅烷醇。机理研究表明,该反应是由 H2O 而不是硅烷激活 FLP 引发的,并通过协同 SN2 机理进行。更重要的是,FLP 催化的氢硅烷氧化反应与 B(C6F5)3 催化的脱氢反应相结合,实现了序列控制型低聚硅氧烷的精确合成。这种方法的底物范围广,易于放大,因此在含硅聚合物材料的精确合成方面具有广阔的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
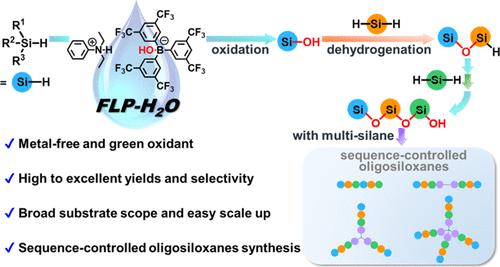
Frustrated Lewis Pair-Promoted Organocatalytic Transformation of Hydrosilanes into Silanols with Water Oxidant
Owing to their unique properties, the silanols have attracted intense attention but remain challenging to prepare from the organocatalytic oxidation of hydrosilanes using H2O as a green oxidant. Herein, we employ a frustrated Lewis pair (FLP) to successfully suppress the formation of undesired siloxanes and produce silanols in high to excellent yields in the presence of H2O. Mechanistic studies suggest that the reaction is initiated with the activation of FLP by H2O rather than by silanes and goes through a concerted SN2 mechanism. More importantly, the combination of the FLP-catalyzed oxidation of hydrosilanes with B(C6F5)3-catalyzed dehydrogenation enables us to realize the precise synthesis of sequence-controlled oligosiloxanes. This method exhibits a broad substrate scope and can be easily scaled up, thus exhibiting promising application potentials in the precision synthesis of silicon-containing polymer materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


