含有 1,2-亚苯基双(3-氯丙酸)取代基团的酞菁锌的合成及其代谢酶抑制作用的研究
IF 2.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
在这项研究中,4,5-二氰基-1,2-亚苯基二烟酸化合物是 4,5-二氯邻苯二甲腈与烟酸反应的结果。该化合物与氯化锌盐反应后,得到了带有 1,2-亚苯基双(3-氯丙酸)取代基团的原始酞菁锌化合物。利用 1H NMR、IR、UV-vis 和质谱对该化合物及其起始材料进行了表征。在化合物(3)和(4)与各目标物(AChE、BChE、α-Amy 和 α-Gly)的对接研究中,与配体结构(3)相比,锌配合物(4)与目标物模型的结合亲和力更强。尤其是 AChE 蛋白与复合物(4)的结合能值为-10.55 kcal/mol,结合亲和力最佳。这些结果与体外分析所获得的数据相吻合,并提供了支持。这些 4,5-二氰基-1,2-亚苯基二烟酸锌(3)和 2, 10, 16, 24-四(1,2-亚苯基双(3-氯丙酸)酞菁)锌(II)(4)复合物对α-葡萄糖苷酶、α-淀粉酶、丁酰胆碱酯酶和 AChE 具有有效的抑制作用。此外,AChE 的 IC50 值分别为 7.84 和 12.36 µM,BChE 的 IC50 值分别为 3.80 和 4.56 µM,α-淀粉酶的 IC50 值分别为 27.08 和 38.14 µM,α-葡萄糖苷酶的 IC50 值分别为 5.30 和 9.73 µM。本文章由计算机程序翻译,如有差异,请以英文原文为准。
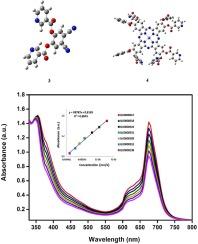
Synthesis of zinc phthalocyanine containing 1,2-phenylene bis(3-chloropropanoate) substituted groups and investigation of their metabolic enzyme inhibitory effects
In this study, 4,5-dicyano-1,2-phenylene dinicotinate compound was obtained as a result of the reaction of 4,5-dichlorophthalonitrile and nicotinic acid. This compound was reacted with the zinc chloride salt to obtain the original zinc phthalocyanine compound bearing 1,2-phenylene bis(3-chloropropanoate) substituted groups. This compound and its starting material were characterized with the assist of 1H NMR, IR, UV–vis, Mass spectrum. In the docking study of compounds (3) and (4) against each target (AChE, BChE, α-Amy and α-Gly), Zn complex (4) exhibits more binding affinity with the target models considered compared to ligand structure (3). Especially, AChE protein and complex (4) form the best binding affinity with a binding energy value of −10.55 kcal/mol. They are compatible and supportive with the data obtained as a result of in vitro analysis. These 4,5-dicyano-1,2-phenylene dinicotinate (3) and 2, 10, 16, 24 – tetrakis 1,2-phenylene bis(3-chloropropanoate) phthalocyaninato) zinc(II) (4) complexes had effective inhibition against α-glucosidase, α-amylase, butyrylcholinesterase and AChE. Also, IC50 amounts were found as 7.84 and 12.36 µM for AChE, 3.80 and 4.56 µM for BChE, 27.08 and 38.14 µM for α-amylase, and 5.30 and 9.73 µM for α-glucosidase.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


