异咯嗪环在自由和蛋白质结合的黄素辅因子中产生单线态氧
IF 3.3
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
黄素辅助因子,即黄素腺嘌呤二核苷酸(FAD)和黄素单核苷酸(FMN),作为黄酶类的一部分,在催化以氧化还原为主的多种反应中发挥着至关重要的作用。人们不禁要问,为什么自然界会产生两种非常相似的辅助因子,而且其功能部分--异咯嗪环--完全相同。我们认为,答案与以下事实有关:异氮杂环属于内源光敏剂,能够高效地产生具有活性和潜在危害的单线态氧 1O2(ΦΔ,FMN∼ 0.6)。事实上,与 FMN 在水中的一种主要构象不同,FAD 中腺苷单核苷酸的存在导致了两种主要构象的动态平衡--封闭构象(∼80%)和开放构象(∼20%)。FAD 在水中的主要封闭构象对ΦΔ,FAD 值有显著影响,ΦΔ,FAD ∼ 0.07,比 FMN 低近 10 倍。另一方面,根据我们对含有 FAD 的 105 个蛋白质的非同源数据集的分析,75% 的酶结合 FAD 主要以开放构象存在,但其 ΦΔ 值显著降低,Φ Δ < 0.03。针对这些相互矛盾的观察结果,我们进行了分析:(i) 脲打开 FAD 构象对ΦΔ,FAD 值的依赖性;(ii) 异丙嗪结合位点的氨基酸倾向性。我们证明了在 7 M 与 0 M 尿素中,脲诱导的封闭 FAD 构象的不稳定性导致ΦΔ增加了 ∼ 3 倍,证明了ΦΔ值与黄素辅助因子构象之间的因果关系。对黄素蛋白数据集的详细研究清楚地表明,甘氨酸、半胱氨酸和色氨酸这三种氨基酸对于异咯嗪环结合位点具有积极的倾向性。我们推测,游离 FAD 的封闭构象和异丙嗪环结合位点的排列对于防止细胞中产生潜在有害的 1O2 非常重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
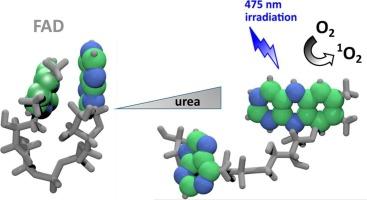
On the production of singlet oxygen by the isoalloxazine ring in free and protein-bound flavin cofactors
Flavin cofactors, flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN), as a part of flavoenzymes play a critical role in the catalysis of multiple reactions predominantly of a redox nature. Question arises why nature developed two very similar cofactors with an identical functional part – isoalloxazine ring. We believe that an answer is related to the fact that the isoalloxazine ring belongs to endogenous photosensitizers able to produce reactive and potentially harmful singlet oxygen, 1O2, with high efficiency, ΦΔ,FMN ∼ 0.6. In fact, in contrast with one main conformation of FMN in water, the presence of the adenosine mononucleotide in FAD induces a dynamic equilibrium of two main conformations – closed (∼80 %) and open (∼20 %). The presence of predominant closed conformation of FAD in water has a significant impact on the ΦΔ,FAD value, which is nearly 10-fold lower, ΦΔ,FAD ∼ 0.07, than that of FMN. On the other hand, based on our analysis of a non-homologous dataset of FAD containing 105 proteins, ∼75 % enzyme-bound FAD exists predominantly in open conformations but the ΦΔ values are significantly decreased, ΦΔ < 0.03. We addressed these contradictory observations by analysis of: (i) dependence of ΦΔ,FAD value on opening the FAD conformation by urea and (ii) amino acid propensities for isoalloxazine binding site. We demonstrated that urea-induced destabilization, in 7 M vs 0 M urea, of the closed FAD conformation leads to a ∼ 3-fold increase of ΦΔ, proving the causative relation between ΦΔ value and the flavin cofactor conformation. Detailed examination of the flavoproteins dataset clearly indicated positive propensities of three amino acids: glycine, cysteine, and tryptophan for isoalloxazine ring binding site. We hypothesize that both the closed conformation of free FAD and the arrangement of the isoalloxazine binding site is important for prevention of potentially harmful 1O2 production in cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


