合成 N-烯丙基-N-苯基氨基甲酸甲酯及其转化为 4,5-二氢异噁唑衍生物
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在液-液相转移催化下,芳香族氨基甲酸酯与溴化烯丙基烷基化反应生成了相应的芳香族氨基甲酸酯 N-烯丙基衍生物,收率为 63-71%。研究发现,在氯胺 T 的存在下,由相应的肟原位生成的炔碳腈 N-氧化物与烯丙基片段在乙醇中沸腾后进行环加成反应,可生成相应的 4,5-二氢异噁唑衍生物,收率为 89-96%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
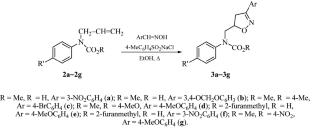
Synthesis of Methyl (Hetarylalkyl) N-Allyl-N-phenylcarbamates and Their Transformation into 4,5-Dihydroisoxazole Derivatives
Alkylation of aromatic carbamates with allyl bromide under liquid-liquid phase transfer catalysis furnished the corresponding N-allyl derivatives of aryl carbamates in 63–71% yields. It was found that the cycloaddition of arene carbonitrile N-oxides, generated in situ from the corresponding oximes in the presence of chloramine T, to the allyl fragment upon boiling in ethanol led to the production of the corresponding 4,5-dihydroisoxazole derivatives in 89–96% yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


