由含有咪唑分子的 β-咔啉衍生的新型季铵盐作为血管生成抑制剂的合成、药理评估和模型制作
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
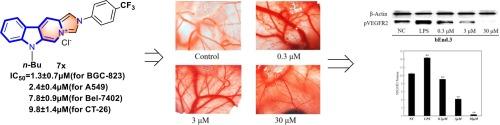
本研究通过在β-咔啉中加入咪唑盐结构,设计并合成了一系列新型β-咔啉缩合咪唑鎓衍生物(7a-7y)。利用 MTT 试验评估了化合物 7a-7y 在各种癌细胞系(包括肺癌(A549)、胃癌(BGC-823)、小鼠结肠癌(CT-26)、肝癌(Bel-7402)和乳腺癌(MCF-7))中的细胞毒性。大多数化合物对一种或多种癌细胞株都有明显的活性。值得注意的是,化合物 7 g、7o、7r、7 s、7u、7v、7x 和 7w 在测试的肿瘤细胞系中显示出最高的细胞毒性活性(IC50 < 2 μM)。化合物 7x 的细胞毒性活性为 1.3 ± 0.3 μM(针对 BGC-823)、2.4 ± 0.4 μM(针对 A549)、7.8 ± 0.9 μM(针对 Bel-7402)和 9.8 ± 1.4 μM(针对 CT-26)。小鸡绒毛膜试验显示化合物 7x 具有显著的抗血管生成潜力。分子印迹研究表明,化合物 7x 的抗血管生成作用可能归因于对 VEGFR2 激酶的抑制。分子对接和分子动力学进一步表明,其活性可能主要与潜在的 VEGFR2 抑制作用有关。我们的研究成果为新型抗肿瘤药物的开发提供了有价值的先导化合物,并为后续的药物设计和优化提供了有益的启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis, pharmacological evaluation, and modeling of novel quaternary ammonium salts derived from β-carboline containing an imidazole moiety as angiogenesis inhibitors
In this study, a series of novel β-carboline condensed imidazolium derivatives (7a-7y) were designed and synthesized by incorporating imidazolium salt structures into β-carboline. The cytotoxicity of compounds 7a-7y was evaluated in various cancer cell lines, including lung cancer (A549), gastric cancer (BGC-823), mouse colon cancer (CT-26), liver cancer (Bel-7402), and breast cancer (MCF-7), using the MTT assay. Most compounds exhibited significant activity against one or more of the cancer cell lines. Notably, compounds 7 g, 7o, 7r, 7 s, 7u, 7v, 7x, and 7w showed the highest cytotoxic activity (IC50 < 2 μM) in the tested tumor cell lines. Compound 7x demonstrated cytotoxic activities of 1.3 ± 0.3 μM (for BGC-823), 2.4 ± 0.4 μM (against A549), 7.8 ± 0.9 μM (for Bel-7402), and 9.8 ± 1.4 μM (against CT-26). The chick chorioallantoic membrane assay revealed significant anti-angiogenic potential of compound 7x. Molecular imprinting studies suggested the anti-angiogenic effect of compound 7x might be attributed to inhibition of VEGFR2 kinase. Molecular docking and molecular dynamics further indicate that its activity may be primarily associated with the potential inhibition of VEGFR2. Our research outcomes have provided valuable lead compounds for the development of novel antitumor drugs and have offered beneficial insights for subsequent drug design and optimization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


