2- 金属萘化学的最新进展:2-碲萘、2-链烷萘、2-链烷萘、2-锗萘及其类似物
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
苯与 4H-四氢吡喃、4H-链烷、4H-链宾或 4H-锗 2d 基团的 c 边在混合构型中融合形成四种不同的结构:缩合 2-碲萘、2-链烷萘、2-链烷萘和 2-锗萘(2-金属萘)。2-金属萘(金属= Te、Sb、Sn 和 Ge)的特点是在 2-金属萘的第二个位置而不是第一个位置存在 Te、Sb、Sn 和 Ge 原子。据我们所知,目前还没有关于这种支架的专门报道,它主要被用作一种构筑基块。合成 2-碲萘、2-链烷萘、2-锡萘或 2-锗萘及其类似物可通过各种化学途径实现,包括 Sonogashira 偶联反应、亲核加成、有机金属反应、金属取代等方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
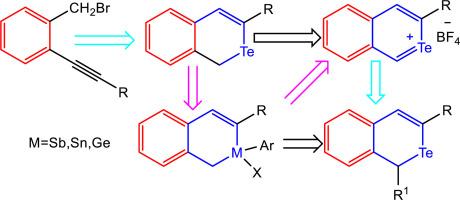
Recent advances in the chemistry of 2-metallanaphthalenes: 2-telluranaphthalene, 2-stibanaphthalene, 2-stannanaphthalene, 2-germanaphthalene and their analogues
The fusion of benzene with the c edge of 4H-telluropyran, 4H-stanine, 4H-stibinine or 4H-germine 2d motifs in hybrid configurations forms four distinct structures: condensed 2-telluranaphthalene, 2-stibanaphthalene, 2-stannanaphthalene and 2-germanaphthalene (2-metallanaphthalenes). 2-Metallanaphthalene (Metal= Te, Sb, Sn and Ge) is characterized by the presence of a Te, Sb, Sn and Ge atom in the second position instead of the first of 2-metallanaphthalene. To our knowledge, no reports have been published specifically on this scaffold, which has primarily been used as a building block. The synthesis of 2-telluranaphthalene, 2-stibanaphthalene, 2-stannanaphthalene or 2-germanaphthalene and their analogues can be achieved through various chemical pathways, including the Sonogashira coupling reactions, nucleophilic addition, organometallic reactions, metal substitution, and other methods.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


