棕色脂肪 ATP-柠檬酸裂解酶将碳水化合物的供应与产热联系起来,并抵御代谢压力
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
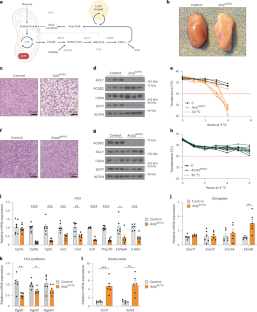
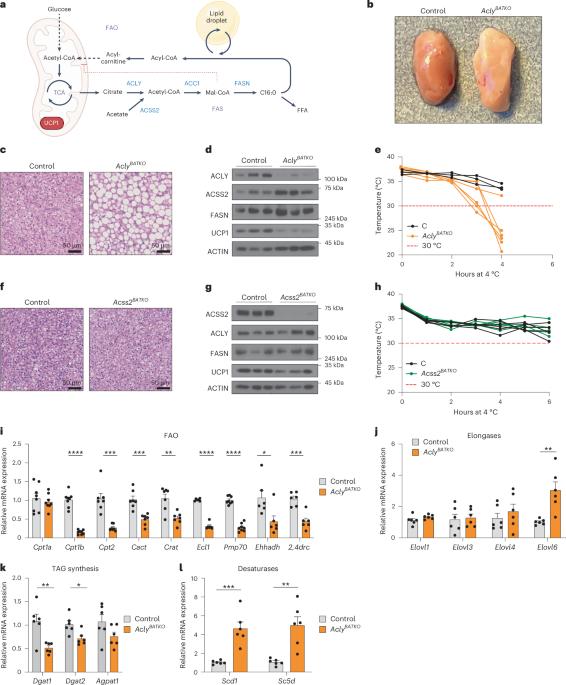
棕色脂肪组织(BAT)进行着徒劳的脂肪酸合成-氧化循环,其目的一直难以捉摸。在这里,我们发现,ATP-柠檬酸裂解酶(ACLY)可生成用于脂肪酸合成的乙酰-CoA,并通过减轻代谢压力来促进产热。如果没有 ACLY,BAT 就会使三羧酸循环超负荷,激活综合应激反应(ISR)并抑制产热。当小鼠断奶后食用富含碳水化合物的食物时,ACLY 在防止 BAT 应激方面的作用就变得至关重要,而去除食物中的碳水化合物则可防止 ACLY 缺乏的 BAT 发生应激反应。缺失 ACLY 还能上调脂肪酸合成酶(Fasn);然而,虽然 ISR 激活本身并不是由脂肪酸合成受损引起的,但删除 Fasn 和 Acly 却能开启另一种代谢程序,从而克服三羧酸循环过载、防止 ISR 激活并挽救产热。总之,我们发现了 ACLY 在减轻线粒体压力方面以前未被认识到的作用,这种作用将膳食碳水化合物与解偶联蛋白 1 依赖性产热联系起来,并为了解 BAT 中脂肪酸合成-氧化悖论提供了基本见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Brown fat ATP-citrate lyase links carbohydrate availability to thermogenesis and guards against metabolic stress
Brown adipose tissue (BAT) engages futile fatty acid synthesis–oxidation cycling, the purpose of which has remained elusive. Here, we show that ATP-citrate lyase (ACLY), which generates acetyl-CoA for fatty acid synthesis, promotes thermogenesis by mitigating metabolic stress. Without ACLY, BAT overloads the tricarboxylic acid cycle, activates the integrated stress response (ISR) and suppresses thermogenesis. ACLY’s role in preventing BAT stress becomes critical when mice are weaned onto a carbohydrate-plentiful diet, while removing dietary carbohydrates prevents stress induction in ACLY-deficient BAT. ACLY loss also upregulates fatty acid synthase (Fasn); yet while ISR activation is not caused by impaired fatty acid synthesis per se, deleting Fasn and Acly unlocks an alternative metabolic programme that overcomes tricarboxylic acid cycle overload, prevents ISR activation and rescues thermogenesis. Overall, we uncover a previously unappreciated role for ACLY in mitigating mitochondrial stress that links dietary carbohydrates to uncoupling protein 1-dependent thermogenesis and provides fundamental insight into the fatty acid synthesis–oxidation paradox in BAT. Korobkina et al. show that ACLY in brown fat prevents metabolic overload in the tricarboxylic acid cycle, thus ensuring adequate thermogenesis by avoiding cellular stress.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


