从超声波数据评估脂肪肝含量的 ANN 不确定性估计
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Computational and structural biotechnology journal
Pub Date : 2024-10-01
DOI:10.1016/j.csbj.2024.09.021
引用次数: 0
摘要
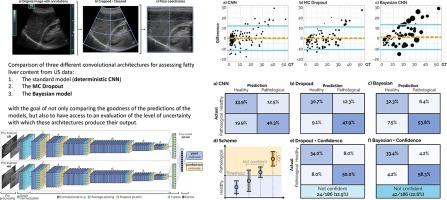
背景和目的本文将三种不同的概率卷积架构应用于超声图像分析,对代谢功能障碍相关性脂肪肝(MASLD)患者的脂肪肝含量(FLC)进行分级。脂肪肝是一种新的无声流行病,其精确测量不仅是肝病专家的迫切临床需求,也是代谢和心血管疾病专家的迫切临床需求。本文旨在对不同的不确定性量化策略进行稳健的比较,以确定在实际临床环境中的优势和缺点。方法我们使用了经典卷积神经网络、蒙特卡洛剔除和贝叶斯卷积神经网络,目的不仅是比较预测的好坏,而且是评估与输出相关的不确定性。结果我们发现,即使基于单个超声波视图的预测是可靠的(相对均方根误差[5.93%-12.04%]),基于两个超声波视图的网络也优于它们(相对均方根误差[5.35%-5.87%])。此外,研究结果表明,引入 "无信心 "类别有助于提高正确预测病例的百分比,降低错误预测病例的百分比,尤其是对于半侵入式方法而言。结论无论是从希望使用神经网络进行计算机辅助诊断的医生的角度,还是从希望限制过拟合和获得有关数据集问题的分布外检测信息的开发人员的角度来看,获取有关网络产生输出结果的信心信息的可能性都是一个巨大的优势。本文章由计算机程序翻译,如有差异,请以英文原文为准。
ANN uncertainty estimates in assessing fatty liver content from ultrasound data
Background and objective
This article uses three different probabilistic convolutional architectures applied to ultrasound image analysis for grading Fatty Liver Content (FLC) in Metabolic Dysfunction Associated Steatotic Liver Disease (MASLD) patients. Steatosis is a new silent epidemic and its accurate measurement is an impelling clinical need, not only for hepatologists, but also for experts in metabolic and cardiovascular diseases. This paper aims to provide a robust comparison between different uncertainty quantification strategies to identify advantages and drawbacks in a real clinical setting.
Methods
We used a classical Convolutional Neural Network, a Monte Carlo Dropout, and a Bayesian Convolutional Neural Network with the goal of not only comparing the goodness of the predictions, but also to have access to an evaluation of the uncertainty associated with the outputs.
Results
We found that even if the prediction based on a single ultrasound view is reliable (relative RMSE [5.93%-12.04%]), networks based on two ultrasound views outperform them (relative RMSE [5.35%-5.87%]). In addition, the results show that the introduction of a “not confident” category contributes to increase the percentage of correctly predicted cases and to decrease the percentage of mispredicted cases, especially for semi-intrusive methods.
Conclusions
The possibility of having access to information about the confidence with which the network produces its outputs is a great advantage, both from the point of view of physicians who want to use neural networks as computer-aided diagnosis, and for developers who want to limit overfitting and obtain information about dataset problems in terms of out-of-distribution detection.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and structural biotechnology journal
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
9.30
自引率
3.30%
发文量
540
审稿时长
6 weeks
期刊介绍:
Computational and Structural Biotechnology Journal (CSBJ) is an online gold open access journal publishing research articles and reviews after full peer review. All articles are published, without barriers to access, immediately upon acceptance. The journal places a strong emphasis on functional and mechanistic understanding of how molecular components in a biological process work together through the application of computational methods. Structural data may provide such insights, but they are not a pre-requisite for publication in the journal. Specific areas of interest include, but are not limited to:
Structure and function of proteins, nucleic acids and other macromolecules
Structure and function of multi-component complexes
Protein folding, processing and degradation
Enzymology
Computational and structural studies of plant systems
Microbial Informatics
Genomics
Proteomics
Metabolomics
Algorithms and Hypothesis in Bioinformatics
Mathematical and Theoretical Biology
Computational Chemistry and Drug Discovery
Microscopy and Molecular Imaging
Nanotechnology
Systems and Synthetic Biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


