Cu(111)支撑的 W3Ox 簇:化学计量和对称性对二氧化碳活化和解离的影响
IF 3.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
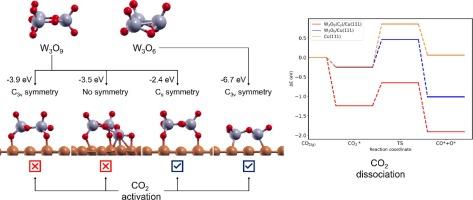
为了研究二氧化碳在 W3Ox/Cu(111)反相催化剂(x = 9 或 6)上的吸附和解离情况,我们进行了带色散修正的密度泛函计算(DFT-D)。W3O9 聚集体通过形成 O-Cu 键以几种不同的几何形状吸附,在所有情况下都从金属表面吸收电子电荷。还原的 W3O6 粒子通过 W-Cu 键与铜紧密结合;在这种情况下,电荷转移与 W3O9/Cu 相反,氧化物粒子带正电。二氧化碳在 W3O6/Cu(111)的氧化物/金属界面上被激活;与纯净的对应物、Cu(111) 和 WO3(001) 表面相比,二氧化碳的解离是放热的,动力学上更有利。相反,二氧化碳在 W3O9/Cu(111)上仅以迄今为止最不稳定的形式(具有 Cs 对称性的形式)被激活。我们的研究结果表明,Cu 支持的 W3Ox 团簇的配量和对称性在二氧化碳的活化和解离中起着至关重要的作用。特别是,W3O6/Cu(111)混合体系在涉及二氧化碳解离的反应中似乎是一种极具潜力的催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cu(111)-supported W3Ox clusters: Stoichiometry and symmetry effects on CO2 activation and dissociation
Density functional calculations with dispersion corrections (DFT-D) have been performed to study the adsorption and dissociation of CO2 on W3Ox/Cu(111) inverse catalyst (x = 9 or 6). The W3O9 aggregate adsorbs in several different geometries through the formation of O-Cu bonds, in all the cases taking electronic charge from the metal surface. The reduced W3O6 particle anchors very strongly to Cu by means of W-Cu bonds; in this case, the charge transfer is opposite than for W3O9/Cu yielding the oxide particle positively charged. CO2 is activated on W3O6/Cu(111) at the oxide/metal interface; its dissociation was found to be exothermic and kinetically more favorable than on the pure counterparts, Cu(111) and WO3(001) surfaces. In contrast, CO2 is activated on W3O9/Cu(111) only in the form that is by far the least stable (the one possessing Cs symmetry). Our results suggest that stoichiometry and symmetry of Cu-supported W3Ox clusters play a crucial role in CO2 activation and dissociation. In particular, the mixed W3O6/Cu(111) system appears as a catalyst of great potential for reactions involving CO2 dissociation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational Materials Science
工程技术-材料科学:综合
CiteScore
6.50
自引率
6.10%
发文量
665
审稿时长
26 days
期刊介绍:
The goal of Computational Materials Science is to report on results that provide new or unique insights into, or significantly expand our understanding of, the properties of materials or phenomena associated with their design, synthesis, processing, characterization, and utilization. To be relevant to the journal, the results should be applied or applicable to specific material systems that are discussed within the submission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


