掺杂杂原子的分层多孔碳对 MgH2 储氢性能的影响
IF 3.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
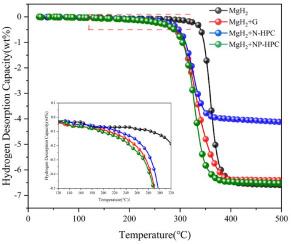
本文通过交联植酸和聚吡咯/苯胺前驱体,分别制备了氮掺杂(N-HPC)、氮磷共掺杂(NP-HPC)分层多孔碳。通过高能球磨将它们与 MgH2 混合,然后研究它们对 MgH2 吸氢和解吸性能的影响和机制。同时,还比较了加入石墨(G)的 MgH2 的储氢性能。结果表明,添加 NP-HPC、N-HPC 和 G 对 MgH2 的吸氢和解吸都有催化作用。在氢气解吸方面,催化效果依次为 N-HPC、G 和 NP-HPC。与纯 MgH2 相比,氢气解吸温度分别降低了 65.3 ℃、79.6 ℃ 和 91.1 ℃。其中,MgH2 + NP-HPC 体系可在 300 °C 温度下于 30 分钟内释放 5.17 wt% 的氢气。第一性原理计算显示,掺杂 P 和含空位的碳材料大大降低了来自 MgH2 表面的 H2 重组势垒,并扭曲了 MgH2 近表面层的原子结构,从而削弱了 Mg-H 键的强度。这可能是 NP-HPC 和含空位 G 对 MgH2 的氢解吸性能具有优异催化效果的内在原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effects of heteroatom-doped hierarchical porous carbon on hydrogen storage properties of MgH2
In this paper, the nitrogen doped (N-HPC), nitrogen and phosphorus co-doped hierarchical porous carbon (NP-HPC) are prepared by cross-linking phytic acid and poly pyrrole/aniline precursor, respectively. They are mixed with MgH2 by high-energy ball milling, and then their effects and mechanisms on the hydrogen absorption and desorption properties of MgH2 are investigated. Meanwhile, the hydrogen storage properties of MgH2 added with graphite (G) are also compared. The results show that the additions of NP-HPC, N-HPC, and G all exhibit the catalytic effect on the hydrogen absorption and desorption of MgH2. As for the hydrogen desorption, the catalytic effect is enhanced in the order of N-HPC, G and NP-HPC. Compared with pure MgH2, the hydrogen desorption temperature is reduced by 65.3 °C, 79.6 °C and 91.1 °C, respectively. Among them, the MgH2 + NP-HPC system can release 5.17 wt% hydrogen at 300 °C within 30 min. First-principles calculations reveal that the P-doped and vacancy-containing carbon materials significantly reduce the H2 recombination barrier from the surface of MgH2 and distort the atomic structure of near-surface layer of MgH2, which in turn weakens the Mg-H bond strength. This may be the intrinsic reason for the excellent catalytic effect of NP-HPC and vacancy-containing G on the hydrogen desorption performance of MgH2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Solid State Sciences
化学-无机化学与核化学
CiteScore
6.60
自引率
2.90%
发文量
214
审稿时长
27 days
期刊介绍:
Solid State Sciences is the journal for researchers from the broad solid state chemistry and physics community. It publishes key articles on all aspects of solid state synthesis, structure-property relationships, theory and functionalities, in relation with experiments.
Key topics for stand-alone papers and special issues:
-Novel ways of synthesis, inorganic functional materials, including porous and glassy materials, hybrid organic-inorganic compounds and nanomaterials
-Physical properties, emphasizing but not limited to the electrical, magnetical and optical features
-Materials related to information technology and energy and environmental sciences.
The journal publishes feature articles from experts in the field upon invitation.
Solid State Sciences - your gateway to energy-related materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


