CYP2E1 在 HepG2 细胞中的亚细胞表达影响对游离油酸和棕榈酸的反应
IF 2.9
Q2 TOXICOLOGY
引用次数: 0
摘要
目的Cytochrome P450 2E1 (CYP2E1) 是一种在肝脏中高水平表达的哺乳动物单加氧酶,可代谢低分子量污染物和药物以及内源性脂肪酸和酮。虽然对 CYP2E1 的研究主要集中在内质网(ER,微粒体部分),但它也大量存在于线粒体中,而对线粒体的研究则少得多。我们研究了线粒体、内质网或两种细胞器中表达 CYP2E1 对暴露于游离油酸和棕榈酸的转基因 HepG2 细胞的影响,包括对细胞毒性、脂质储存、呼吸和基因表达的影响。CYP2E1 的表达不会影响脂肪酸暴露引起的脂质积累,但线粒体 CYP2E1 的表达会促进血清饥饿时的脂滴耗竭。与 HepG2 细胞相反,分化的 HepaRG 细胞表达大量的 CYP2E1,但它们对棕榈酸的细胞毒性并不敏感。油酸暴露引起的细胞毒性较小,ER 中 CYP2E1 的表达阻止了油酸诱导的呼吸作用的增加。接触棕榈酸和油酸混合物的 HepG2 细胞可免受棕榈酸的细胞毒性。此外,我们还发现肝细胞癌中的 CYP2E1 在基因和蛋白质水平上都有所下降。此外,与 CYP2E1 表达较低的患者相比,CYP2E1 表达较高的肿瘤患者预后较好。这项研究表明,转基因 CYP2E1 亚细胞定位在暴露于棕榈酸和油酸的肝癌细胞系 HepG2 的细胞毒性敏感性、脂质储存和呼吸中起着重要作用。相反,HepaRG 细胞对棕榈酸不敏感。这项工作证明了 CYP2E1 在决定脂肪毒性方面的明显重要性,以及线粒体和 ER 形式的酶的不同作用。结论:CYP2E1 在改变脂肪毒性方面发挥着作用,由于已知 CYP2E1 在肝病和肝细胞癌中都会上调,因此更好地确定 CYP2E1 在疾病进展过程中的作用如何变化非常重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
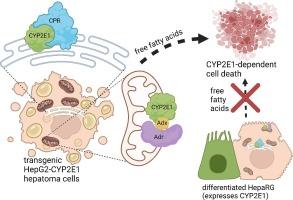
Subcellular expression of CYP2E1 in HepG2 cells impacts response to free oleic and palmitic acid
Aims
Cytochrome P450 2E1 (CYP2E1) is a mammalian monooxygenase expressed at high levels in the liver that metabolizes low molecular weight pollutants and drugs, as well as endogenous fatty acids and ketones. Although CYP2E1 has been mainly studied in the endoplasmic reticulum (ER, microsomal fraction), it also localizes in significant amounts to the mitochondria, where it has been far less studied. We investigated the effects of CYP2E1 expression in mitochondria, endoplasmic reticulum, or both organelles in transgenic HepG2 cells exposed to free oleic and palmitic acid, including effects on cytotoxicity, lipid storage, respiration, and gene expression.
Results
We found that HepG2 cells expressing CYP2E1 in both the ER and mitochondria have exacerbated levels of palmitic acid cytotoxicity and inhibited respiration. CYP2E1 expression did not impact lipid accumulation from fatty acid exposures, but mitochondrial CYP2E1 expression promoted lipid droplet depletion during serum starvation. In contrast to HepG2 cells, differentiated HepaRG cells express abundant CYP2E1, but they are not sensitive to palmitic acid cytotoxicity. Oleic acid exposure prompted less cytotoxicity, and CYP2E1 expression in the ER prevented an oleic-acid-induced increase in respiration. HepG2 cells exposed to mixtures of palmitic and oleic acid are protected from palmitic acid cytotoxicity. Additionally, we identified that CYP2E1 was decreased at the gene and protein level in hepatocellular carcinoma. Moreover, patients with tumors that had higher CYP2E1 expression had a better prognosis compared to patients with lower CYP2E1 expression.
Innovation
This study has demonstrated that transgenic CYP2E1 subcellular localization plays an important role in sensitivity to cytotoxicity, lipid storage, and respiration in the hepatoma cell line HepG2 exposed to palmitic and oleic acid. HepaRG cells, in contrast, were insensitive to palmitic acid. This work demonstrates the clear importance of CYP2E1 in dictating lipotoxicity and differential roles for the mitochondrial and ER forms of the enzyme. Additionally, our data supports a potentially unique role for CYP2E1 in cancer cells.
Conclusion
There lies a role for CYP2E1 in altering lipotoxicity, and since CYP2E1 is known to be upregulated in both liver disease and hepatocellular carcinoma, it is important to better define how the role of CYP2E1 changes during disease progression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current Research in Toxicology
Environmental Science-Health, Toxicology and Mutagenesis
CiteScore
4.70
自引率
3.00%
发文量
33
审稿时长
82 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


