多孔有机纳米笼 CC1 对 H2S、SF6、SF4、SOCl2 和 SO2 的奇特捕集行为;DFT 观点
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
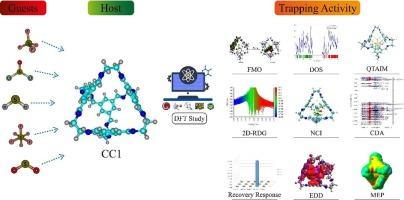
目前的研究探讨了利用多孔有机笼 CC1(一种具有可触及空隙的共价结合亚胺笼)捕集有害气体的问题。研究采用 WB97XD/6-311G(d,p)理论水平,计算了 CC1 及其与 H2S、SF6、SF4、SOCl2 和 SO2 等污染物的配合物。在 DFT/ B3LYP/6-311G(d, p) 理论水平上进行了 DOS 和 FMO 分析。相互作用能、NCI、QTAIM、EDD、NBO、电荷离解、FMO、DOS 和 MEP 研究为分析物 @CC1 复合物提供了详细的见解。相互作用能(-4.49 至 -0.34 eV)表明,SOCl2 和 SF4 有很强的捕获能力,而 H2S 在 CC1 的空腔内有轻微的捕获能力。热力学参数证实了这些发现。分析(NCI、QTAIM)显示出强烈的非共价相互作用,尤其是 SOCl2 和 SF4。NBO 分析显示,除 H2S 外,分析物的电荷都转移到了保持架上。EDD 分析也用于验证 NBO 电荷转移。这项研究强调了 CC1 对所考虑的分析物的特殊捕获行为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Peculiar trapping behavior of porous organic nanocage CC1 towards H2S, SF6, SF4, SOCl2 and SO2; A DFT Perspective
The current study explores the trapping of harmful gases using porous organic cage CC1, a covalently bound imine cage with accessible voids. Using WB97XD/6-311G(d, p) theory level, the research computes CC1 and its complexes with pollutants like H2S, SF6, SF4, SOCl2, and SO2. DOS and FMO analyses are performed at DFT/ B3LYP/6-311G(d, p) theoretical level. Interaction energy, NCI, QTAIM, EDD, NBO, charge dissociation, FMO, DOS, and MEP studies provide a detailed insight into the analytes@CC1 complexation. Interaction energies (−4.49 to −0.34 eV) show strong trapping of SOCl2 and SF4 while, slight trapping of H2S within the cavity of CC1. Thermodynamic parameters confirm these findings. Analyses (NCI, QTAIM) indicate strong non-covalent interactions, especially for SOCl2 and SF4. NBO analysis reveals charge transfer from analytes to the cage, except for H2S. EDD analysis is also implemented to verify NBO charge transfer. This study highlights CC1′s peculiar trapping behaviour for the considered analytes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


