揭示 CXCL8/CXCR2 在椎间盘退变中的作用:通往有前景的治疗策略之路
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
摘要
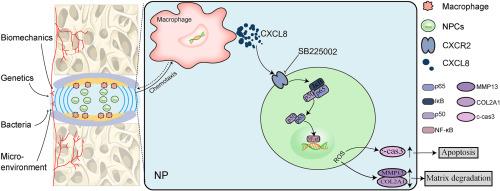
背景椎间盘退变(IVDD)是腰痛和根性痛的主要病因。最近的研究发现趋化因子在 IVDD 中起作用,但其潜在机制尚不清楚。采用 RT-qPCR、Western 印迹(WB)、免疫组织化学(IHC)和酶联免疫吸附试验(ELISA)对 CXCL8 和 CXCR2 的表达水平进行了定量分析。在 IVDD 小鼠模型中,通过 X 射线图像、Safranin O-快绿染色(SO-FG)、IHC 和 WB 来评估 CXCL8 对 IVDD 的治疗效果。通过WB、流式细胞术、免疫荧光(IF)和隧道试验评估了活性氧(ROS)的产生、髓核细胞(NPCs)的凋亡和NF-κB通路的参与。通过中和 CXCL8 或其受体 CXCR2(SB225002,CXCR2 拮抗剂),可明显减轻细胞外基质降解(ECM)和 IVDD 的严重程度。椎间盘(IVDs)内浸润的巨噬细胞在受到刺激时主要释放 CXCL8。CXCL8 通过激活 CXCR2 诱导细胞凋亡和 ECM 降解,从而对 NPCs 发挥作用。具体来说,CXCL8/CXCR2 复合物的形成触发了 NF-κB 信号通路,导致细胞内 ROS 水平异常升高,最终导致 IVDD 的发生。靶向 CXCL8/CXCR2 轴可能为改善 IVDD 提供有前景的治疗策略。本研究表明,CXCL8 可通过激活 NF-κB 通路有效加剧 NPC 的过度凋亡和氧化应激。这项研究可能为预防和逆转 IVDD 提供新的潜在靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unveiling the role of CXCL8/CXCR2 in intervertebral disc degeneration: A path to promising therapeutic strategies
Background
Intervertebral disc degeneration(IVDD) is the primary etiology of low back pain and radicular pain. Recent studies have found that chemokines play a role in IVDD, but the underlying mechanism is largely unclear.
Methods
Bioinformatics analysis was employed to screen CXCL8 as the target gene. The expression levels of CXCL8 and CXCR2 were quantified using RT-qPCR, western blot(WB), immunohistochemistry(IHC), and enzyme-linked immuno-sorbent assay(ELISA). In the IVDD mouse model, X-ray images, Safranin O-fast green staining(SO-FG), IHC, and WB were conducted to assess the therapeutic effects of CXCL8 on IVDD. Reactive oxygen species (ROS) production, apoptosis of nucleus pulposus cells (NPCs), and the involvement of the NF-κB pathway were evaluated through WB, flow cytometry, immunofluorescence(IF), and Tunnel assay.
Results
In our study, we observed that CXCL8 emerged as one of the chemokines that were up-regulated in IVDD. The mitigation of extracellular matrix degradation (ECM) and the severity of IVDD were significantly achieved by neutralizing CXCL8 or its receptor CXCR2(SB225002, CXCR2 antagonist). The release of CXCL8 from infiltrated macrophages within intervertebral discs (IVDs) was predominantly observed upon stimulation. CXCL8 exerted its effects on NPCs by inducing apoptosis and ECM degradation through the activation of CXCR2. Specifically, the formation of the CXCL8/CXCR2 complex triggered the NF-κB signaling pathway, resulting in an abnormal increase in intracellular ROS levels and ultimately contributing to the development of IVDD.
Conclusion
Our findings suggest that macrophage-derived CXCL8 and subsequent CXCR2 signaling play crucial roles in mediating inflammation, oxidative stress, and apoptosis in IVDD. Targeting the CXCL8/CXCR2 axis may offer promising therapeutic strategies to ameliorate IVDD.
The translational potential of this article
This study indicates that CXCL8 can effectively exacerbate the excessive apoptosis and oxidative stress of NPCs through activating the NF-κB pathway. This study may provide new potential targets for preventing and reversing IVDD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


