CD163/TWEAK/Fn14轴:缓解炎症性骨质流失的潜在治疗靶点
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
摘要
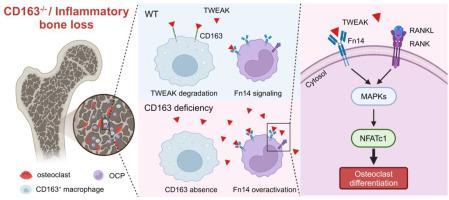
目的破骨细胞(OC)过度激活是骨质流失的一个重要原因,它与炎症密切相关。尽管 CD163/TWEAK/Fn14 轴与多种炎症性病变有关,但人们对其在炎症性骨质流失中的作用仍知之甚少。本研究旨在评估 CD163/TWEAK/Fn14 轴与 OC 在炎症性骨质流失中的相互作用。方法为了评估 CD163 在骨稳态中的作用,我们对 CD163 缺失小鼠及其野生型同窝小鼠的骨表型进行了鉴定。对LPS诱导骨质流失的小鼠和类风湿性关节炎(RA)患者骨髓中的CD163和TWEAK水平进行了评估。在CD163缺陷小鼠和LPS诱导骨质流失小鼠体内补充重组小鼠CD163蛋白(rCD163)或阻断TWEAK/Fn14信号传导后,使用uCT和组织学方法评估骨质变化。在体外分析了 CD163/TWEAK 对 OC 分化和骨吸收能力的影响。在 LPS 诱导骨质流失的小鼠和 RA 患者的骨髓中观察到较低的 CD163 表达和较高的 TWEAK 表达。TWEAK主要来源于CD68+巨噬细胞,是造成骨质流失的原因,补充rCD163或阻断TWEAK/Fn14信号有助于挽救骨质流失。TWEAK/Fn14通过下游丝裂原活化蛋白激酶(MAPK)信号协同促进了RANKL依赖性OC分化和骨吸收能力,而CD163抑制了TWEAK的促破骨细胞作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The CD163/TWEAK/Fn14 axis: A potential therapeutic target for alleviating inflammatory bone loss
Objective
Osteoclast (OC) over-activation is an important cause of bone loss that is strongly correlated with inflammation. Although the CD163/TWEAK/Fn14 axis has been implicated in several inflammatory pathologies, its contributions to inflammatory bone loss remain poorly understood. This study aimed to evaluate the interaction of the CD163/TWEAK/Fn14 axis with OC in inflammatory bone loss.
Methods
To assess the role of CD163 in bone homeostasis, we characterized the bone phenotypes of CD163-deficient mice and their wild-type littermates. CD163 and TWEAK levels were evaluated in the bone marrow of mice with LPS-induced bone loss and individuals with rheumatoid arthritis (RA). Bone mass changes were assessed using uCT and histology following supplementation with recombinant mouse CD163 protein (rCD163) or blockade of TWEAK/Fn14 signaling in CD163-deficient mice and mice with LPS-induced bone loss. The impact of CD163/TWEAK on OC differentiation and bone resorption capacity was analyzed in vitro.
Results
CD163 deficiency caused decreased bone mass and increased OC abundance. Lower CD163 expression and higher TWEAK expression were observed in the bone marrow of mice with LPS-induced bone loss and individuals with RA. TWEAK, mainly derived from CD68+ macrophages, was responsible for bone loss, and supplementing rCD163 or blocking TWEAK/Fn14 signaling contributed to rescue bone loss. TWEAK/Fn14 synergistically promoted RANKL-dependent OC differentiation and bone resorption capability through downstream mitogen-activated protein kinases (MAPK) signaling, while the pro-osteoclastic effect of TWEAK was suppressed by CD163.
Conclusion
Our findings suggest that the CD163/TWEAK/Fn14 axis is a potential therapeutic target for inflammatory bone loss by regulating osteoclastogenesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


