人脐带间充质干细胞衍生的外泌体通过 miR-132-3p/FoxO3 轴改善肌肉萎缩状况
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
摘要
背景肌肉萎缩或肌肉疏松症是指肌肉质量和力量的丧失,会导致残疾和死亡风险增加,包括骨质疏松性骨折。目前,还没有治疗肌肉疏松症的临床生物制剂。由于外泌体能够促进蛋白质和核糖核酸的细胞间转移,促进细胞修复和功能恢复,因此作为一种新型治疗方法越来越具有吸引力,我们假设人脐带间充质干细胞衍生的外泌体(hucMSC-Exos)可能对年龄相关性和地塞米松诱导的肌肉疏松症动物模型的肌肉萎缩有益。方法 通过超速离心法收获 hucMSC-Exos,并通过透射电子显微镜、粒度分析和 Western 印迹分析进行鉴定。使用年龄相关和地塞米松诱导的肌肉萎缩小鼠模型评估了 hucMSC-Exos 对肌肉萎缩的影响。对这些小鼠的体重、握力、肌肉重量和肌肉组织学进行了评估。用 Western 印迹法测定了肌肉环指 1(MuRF1)和肌肉萎缩 F-box(atrogin-1)的表达水平。地塞米松诱导的C2C12肌管萎缩被用来建立肌肉萎缩的细胞模型。肌管直径通过免疫荧光染色进行评估。结果在体内实验中,hucMSC-Exos能显著改善老年性肌肉萎缩小鼠和地塞米松诱导的肌肉萎缩小鼠的握力、增加肌肉质量和肌纤维横截面积,同时降低MuRF1和atrogin-1的表达。在体外实验中,hucMSC-Exos 能促进 C2C12 细胞的增殖,并能挽救地塞米松诱导的 C2C12 肌管活力下降。此外,hucMSC-Exos还能增加C2C12肌管的直径,并降低地塞米松诱导的MuRF1和atrogin-1的上调。结合生物信息学分析和 RNA 测序分析,我们进一步发现 miR-132-3p 是 hucMSC-Exos 中必不可少的 miRNA 之一,并通过靶向 FoxO3 发挥重要作用。本研究首次证明了 hucMSC-Exos 可通过 miR-132-3p/FoxO3 轴改善肌肉萎缩。这些数据可为 hucMSC-Exos 治疗肌肉疏松症的临床转化提供新颖而有价值的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
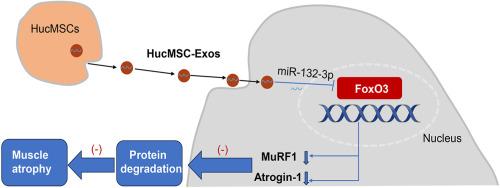
Human umbilical cord mesenchymal stem cell-derived exosomes ameliorate muscle atrophy via the miR-132-3p/FoxO3 axis
Background
Muscle atrophy or sarcopenia is the loss of muscle mass and strength and leads to an increased risk of disability and death including osteoporotic fractures. Currently, there are no available clinical biologic agents for the treatment of sarcopenia. Since exosomes have become increasingly attractive as a novel therapeutic approach due to their ability to facilitate cell-cell transfer of proteins and RNAs, promoting cell repair and function recovery, we hypothesized that human umbilical cord mesenchymal stem cell-derived exosomes (hucMSC-Exos) might benefit muscle atrophy in age-related and dexamethasone-induced sarcopenia animal models.
Methods
HucMSC-Exos were harvested by ultrafast centrifugation and identified by transmission electron microscopy, particle size analysis, and Western blot analysis. The effects of hucMSC-Exos on muscle atrophy were evaluated using age-related and dexamethasone-induced muscle atrophy mice models. Body weight, grip strength, muscle weight, and muscle histology of these mice were assessed. The expression levels of muscle RING finger 1 (MuRF1) and muscle atrophy F-box (atrogin-1) were measured by Western blot. Dexamethasone-induced C2C12 myotube atrophy was used to establish the cell model of muscle atrophy. Myotube diameter was evaluated by immunofluorescence staining. Bioinformatic analysis, RNA sequencing analysis, and Western blot analysis were performed to explore the underlying mechanisms.
Results
In vivo experiments, hucMSC-Exos demonstrated a remarkable capacity to improve grip strength, increase muscle mass, and muscle fiber cross-sectional area, while concurrently reducing the expression of MuRF1 and atrogin-1 in age-related and dexamethasone-induced muscle atrophy mice. In vitro experiments, hucMSC-Exos can promote the proliferation of C2C12 cells, and rescue the dexamethasone-induced decline in the viability of C2C12 myotubes. In addition, hucMSC-Exos can increase the diameter of C2C12 myotubes, and reduce dexamethasone-induced upregulation of MuRF1 and atrogin-1. Combined with bioinformatics analysis and RNA sequencing analysis, we further showed that miR-132-3p was one of the essential miRNAs in hucMSC-Exos and played an important role by targeting FoxO3.
Conclusion
Our findings suggested that hucMSC-Exos can improve age-related and dexamethasone-induced muscle atrophy in mice models. This study first demonstrated that hucMSC-Exos may ameliorate muscle atrophy via the miR-132-3p/FoxO3 axis. These data may provide novel and valuable insights into the clinical transformation of hucMSC-Exos for the treatment of sarcopenia.
The translational potential of this article
HucMSC-Exos are easily available for clinical application, this study further consolidates the evidence for the clinical transformation potential of hucMSC-Exos for sarcopenia and provides its new target pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


