基于截短 NPY 的 NPY(Y1)R 特异性放射多肽:应用肽酶抑制剂改进体内 PET 肿瘤成像
European Journal of Medicinal Chemistry Reports
Pub Date : 2024-10-03
DOI:10.1016/j.ejmcr.2024.100223
引用次数: 0
摘要
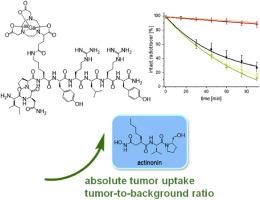
神经肽 Y 受体亚型 1(NPY(Y1)R)在人类乳腺癌中的表达率很高,因此是利用正电子发射断层扫描(PET)灵敏、特异地观察和描述乳腺癌的重要靶结构。然而,由于基于肽的 NPY 衍生 NPY(Y1)R 特异性放射性示踪剂候选物在体内降解导致其稳定性较低,因此该受体类型的成像迄今为止成功率有限。鉴于稳定这些制剂所面临的挑战,我们的研究试图探索能否提高 NPY(Y1)R 特异性放射肽的稳定性。我们的目标是通过使用各种分子支架或使用肽酶抑制剂来改变肽的结构。这项评估的目的是确定实现有效 NPY(Y1)R 特异性成像的最佳方法。为了验证我们的方法,我们系统地研究了四种新的截短 68Ga 标记的 NPY 类似物和参考化合物 [68Ga]Ga-[Lys4(Nε-DOTA)]BVD15,它们具有不同的分子支架,如 DOTA 或 NODA-GA 螯合剂、4-APipAc 连接器、Bip 单元和 N 端 Lys(月桂基)基团。四种新的放射性肽以及参考化合物的化学和放射化学收率都很高,摩尔活性为 33-39 GBq/μmol。这些放射性肽的 logD7.4 值从 -3.37 ± 0.09 到 +0.35 ± 0.11 不等,并且根据其分子结构的不同,在人血清和肝脏微粒体中表现出不同程度的稳定性。随后,研究人员还调查了肽酶抑制剂放线菌素、磷胺、卡托普利和 E-64 对放射性racer 体外稳定性的影响。在这些研究中,只有放线菌素对所有放射性肽的稳定性都有积极影响。因此,在 T47D 肿瘤异种移植小鼠模型中,研究了放线菌素给药对最有前途的配体 [68Ga]Ga-[Lys4(Nε-NODA-GA)]BVD15([68Ga]Ga-2)体内 PET/CT 成像结果的影响,随后进行了体内外生物分布研究。在这些实验中,给予 250 μg 的放线菌素后,T47D 肿瘤对[68Ga]Ga-2 的摄取量明显增加(2 小时后为 5.9 ± 1.0 % ID/g(含放线菌素),而不是 3.1 ± 0.9 % ID/g(不含放线菌素)),肿瘤与肌肉的比率从 1.8 增加到 4.0。这些结果令人印象深刻地证明了放线菌素对 NPY(Y1)R 特异性放射多肽 [68Ga]Ga-2 体内稳定性的积极影响,从而增加了肿瘤蓄积并改善了肿瘤与背景的比率。因此,这些发现为进一步推动使用 PET 进行 NPY(Y1)R 特异性肿瘤成像提供了重要动力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Truncated NPY-based NPY(Y1)R-specific radiopeptides: Improved in vivo PET tumor imaging by application of peptidase inhibitors
The neuropeptide Y receptor subtype 1 (NPY(Y1)R) exhibits high expression rates on human breast cancer and is therefore an important target structure for the sensitive and specific visualization and characterization of the disease by Positron Emission Tomography (PET). However, imaging of this receptor type has been of limited success so far due to the low stability of peptide-based NPY-derived NPY(Y1)R-specific radiotracer candidates due to in vivo degradation. Given the challenges in stabilizing these agents, our study sought to explore whether the stability of NPY(Y1)R-specific radiopeptides could be enhanced. We aimed to achieve this by either modifying the peptide structure with various molecular scaffolds or by applying peptidase inhibitors. This evaluation aimed to identify the optimal approach for achieving effective NPY(Y1)R-specific imaging in the following. To validate our approach, we systematically investigated four new truncated 68Ga-labeled analogs of NPY and the reference compound [68Ga]Ga-[Lys4(Nε-DOTA)]BVD15, bearing different molecular scaffolds such as a DOTA or NODA-GA chelator, a 4-APipAc linker, a Bip unit, and an N-terminal Lys(lauryl) group. The four new radiotracers as well as the reference compound were obtained in high chemical and radiochemical yields with molar activities of 33–39 GBq/μmol. The radiopeptides exhibited varying logD7.4 values, ranging from −3.37 ± 0.09 to +0.35 ± 0.11, and showed different levels of stability in human serum and liver microsomes, depending on their molecular structure. Subsequently, the influence of the peptidase inhibitors actinonin, phosphoramidon, captopril and E−64 on the in vitro stability of the radiotracers was investigated. In these studies, only actinonin demonstrated a positive effect on the stability of all radiopeptides. In contrast, phosphoramidon yielded variable results, and neither captopril nor E−64 showed a significant stabilizing effect.
Consequently, the effect of actinonin administration on the in vivo PET/CT imaging results of the most promising ligand [68Ga]Ga-[Lys4(Nε-NODA-GA)]BVD15 ([68Ga]Ga-2) was investigated in a T47D tumor-bearing xenograft mouse model, followed by ex vivo biodistribution studies. In these experiments, the administration of 250 μg actinonin resulted in a significantly increased uptake of [68Ga]Ga-2 in the T47D tumor (5.9 ± 1.0 % ID/g (with actinonin) instead of 3.1 ± 0.9 % ID/g (without actinonin) at 2h p.i.), and an increase in tumor-to-muscle ratios from 1.8 to 4.0 upon co-administration of the inhibitor.
The results impressively demonstrate the positive influence of actinonin on the in vivo stability of the NPY(Y1)R-specific radiopeptide [68Ga]Ga-2, resulting in an increased tumor accumulation and improved tumor-to-background ratios. These findings thus provide important incentive for further advancement of NPY(Y1)R-specific tumor imaging using PET.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


