添加氧化铜形成 CuO/TiO2 和 CuO/ZnO 异质结,用于光催化 CO2 转化为甲醇
IF 3.1
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
光催化将二氧化碳(CO2)转化为高附加值产品是实现可持续发展的一条大有可为的途径。在这项研究中,利用 TiO2、ZnO、CuO/TiO2 和 CuO/ZnO 光催化剂将存在水蒸气和紫外线照射源的气相二氧化碳转化为甲醇。通过物理化学、光学和基于第一原理的技术进行表征后发现,CuO/TiO2 具有更高的氧空位、最高的平均电子寿命、转移率、注入效率和最高的分离效率。CuO/TiO2 的甲醇产率最高(28%),这是由于异质结的形成和作为电子捕获催化剂的 CuO 掺杂增加了表面氧空位的共同作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
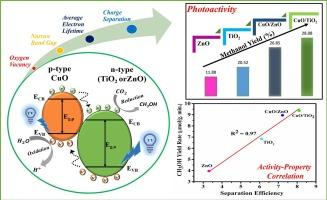
Addition of CuO to form CuO/TiO2 and CuO/ZnO heterojunctions for photocatalytic CO2 conversion to methanol
Photocatalytic conversion of carbon dioxide (CO2) to value-added products is a promising route towards sustainability. In this work, gas phase CO2 in presence of water vapor and UV irradiation source was converted to methanol using TiO2, ZnO, CuO/TiO2 and CuO/ZnO photocatalysts. Characterization by physico-chemical, optical and First-principle based techniques revealed that CuO/TiO2 possessed increased oxygen vacancy, highest average electron lifetime, transfer rate, injection efficiency and highest separation efficiency. The maximum methanol (∼28 %) yield of CuO/TiO2 is ascribed due to combined effect of heterojunction formation and increment in surface oxygen vacancies by doping with CuO, acted as electron-trapping cocatalyst.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics Letters
化学-物理:原子、分子和化学物理
CiteScore
5.70
自引率
3.60%
发文量
798
审稿时长
33 days
期刊介绍:
Chemical Physics Letters has an open access mirror journal, Chemical Physics Letters: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Chemical Physics Letters publishes brief reports on molecules, interfaces, condensed phases, nanomaterials and nanostructures, polymers, biomolecular systems, and energy conversion and storage.
Criteria for publication are quality, urgency and impact. Further, experimental results reported in the journal have direct relevance for theory, and theoretical developments or non-routine computations relate directly to experiment. Manuscripts must satisfy these criteria and should not be minor extensions of previous work.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


