C3-C6 环烷中 NH2 自由基的氢萃取反应:理论研究
IF 2.8
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
氨基(NH2)自由基在氨热解和氧化过程中至关重要,其在 NH3 双燃料燃烧中的反应已得到广泛认可。我们采用 CCSD(T)/aug-cc-pVTZ//M06-2X/aug-cc-pVTZ 理论水平,从理论上研究了 NH2 自由基与 C3-C6 环烷(汽油的一种组分类型)的反应。我们计算出的 NH2 与环己烷反应的速率常数与实验测量值相吻合,但其他反应的实验数据有限。我们还提供了所研究反应的阿伦尼乌斯参数。本文章由计算机程序翻译,如有差异,请以英文原文为准。
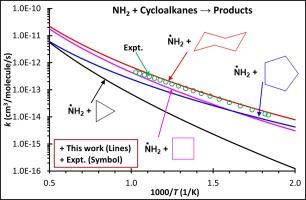
Hydrogen abstraction reactions by NH2 radicals from C3-C6 Cycloalkanes: A theoretical study
Amino (NH2) radicals are crucial in ammonia pyrolysis and oxidation, and their reactions in NH3-dual-fuel combustion are well-recognized. We theoretically investigated the reactions of NH2 radicals with C3-C6 cycloalkanes – a component type of gasoline, using the CCSD(T)/aug-cc-pVTZ//M06-2X/aug-cc-pVTZ level of theory. Canonical transition state theory with corrections for hindered internal rotation and Eckart tunneling effects was employed to gain high-pressure limiting rate constants over 500 – 2000 K. Our calculated rate constant for NH2 with cyclohexane matches the experimental measurement, though experimental data for other reactions are limited. The Arrhenius parameters for the studied reactions are also provided.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics Letters
化学-物理:原子、分子和化学物理
CiteScore
5.70
自引率
3.60%
发文量
798
审稿时长
33 days
期刊介绍:
Chemical Physics Letters has an open access mirror journal, Chemical Physics Letters: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Chemical Physics Letters publishes brief reports on molecules, interfaces, condensed phases, nanomaterials and nanostructures, polymers, biomolecular systems, and energy conversion and storage.
Criteria for publication are quality, urgency and impact. Further, experimental results reported in the journal have direct relevance for theory, and theoretical developments or non-routine computations relate directly to experiment. Manuscripts must satisfy these criteria and should not be minor extensions of previous work.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


