血浆诱导氧化对 NK 细胞免疫检查点配体的影响:计算-实验方法
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
非热等离子体(NTP)是一种有效的抗癌疗法,具有细胞毒性和免疫调节作用。在本研究中,我们研究了 NTP 诱导的氧化对自然杀伤(NK)细胞功能的几个关键、决定性免疫检查点的化学和生物效应。我们使用分子动力学(MD)和伞状取样模拟来研究 NTP 诱导的氧化变化对 MHC-I 复合物 HLA-Cw4 和 HLA-E 的影响。我们的模拟结果表明,这些化学变化并不会明显影响这些标记物与相应的 NK 细胞受体的结合亲和力,这与应用 NTP 后配体在人类头颈部鳞状细胞癌细胞上的表达实验结果相吻合。我们将研究范围扩大到 NK 细胞反应性的其他关键配体,结果表明抑制性 TIGIT 轴的目标配体 CD155 和 CD112 以及免疫检查点 CD73 在治疗后立即迅速减少。除了这些短暂的化学变化外,NTP 中的活性物种还会引起一连串的下游细胞反应。处理后 24 小时,NK 细胞活化的有效配体应激蛋白 MICA/B 的上调凸显了这一点。综上所述,这项研究证实了 NTP 的免疫调节潜力,并揭示了 NTP 与癌细胞之间的相互作用机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
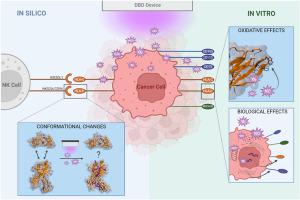
Effect of plasma-induced oxidation on NK cell immune checkpoint ligands: A computational-experimental approach
Non-thermal plasma (NTP) shows promise as a potent anti-cancer therapy with both cytotoxic and immunomodulatory effects. In this study, we investigate the chemical and biological effects of NTP-induced oxidation on several key, determinant immune checkpoints of natural killer (NK) cell function. We used molecular dynamics (MD) and umbrella sampling simulations to investigate the effect of NTP-induced oxidative changes on the MHC-I complexes HLA-Cw4 and HLA-E. Our simulations indicate that these chemical alterations do not significantly affect the binding affinity of these markers to their corresponding NK cell receptor, which is supported with experimental read-outs of ligand expression on human head and neck squamous cell carcinoma cells after NTP application. Broadening our scope to other key ligands for NK cell reactivity, we demonstrate rapid reduction in CD155 and CD112, target ligands of the inhibitory TIGIT axis, and in immune checkpoint CD73 immediately after treatment. Besides these transient chemical alterations, the reactive species in NTP cause a cascade of downstream cellular reactions. This is underlined by the upregulation of the stress proteins MICA/B, potent ligands for NK cell activation, 24 h post treatment. Taken together, this work corroborates the immunomodulatory potential of NTP, and sheds light on the interaction mechanisms between NTP and cancer cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


