HJURP 通过增强 PRDX1 的过氧化物酶活性来抑制前列腺癌细胞对铁变态诱导剂的敏感性
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
铁氧化诱导已成为治疗前列腺癌(PCa)的一种很有前景的方法,既可作为一种单一疗法,也可与激素疗法结合使用。因此,确定 PCa 细胞中的铁突变调控机制至关重要。我们之前的研究表明,PCa 细胞中上调的癌基因 HJURP 在肿瘤增殖中起作用。在这里,我们通过阐明 HJURP 在体外和体内通过 PRDX1/活性氧(ROS)途径抑制 PCa 细胞对铁突变诱导剂的敏感性的新机制,扩展了这些发现。从机理上讲,HJURP 通过 Cys327 和 Cys457 残基与 PRDX1 形成二硫键中间体。这种二硫键结合促进了 PRDX1 的氧化还原循环,抑制了它的高氧化作用。因此,HJURP 能增强 PRDX1 的过氧化物酶活性,导致 ROS 水平下降,进而抑制铁变态诱导剂诱导的脂质过氧化反应。这些发现揭示了 HJURP/PRDX1 作为新的治疗靶点和 PCa 患者铁变态反应生物标志物的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
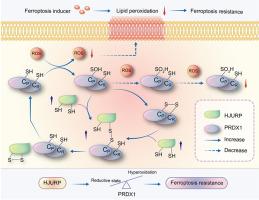
HJURP inhibits sensitivity to ferroptosis inducers in prostate cancer cells by enhancing the peroxidase activity of PRDX1
Ferroptosis induction has emerged as a promising therapeutic approach for prostate cancer (PCa), either as a monotherapy or in combination with hormone therapy. Therefore, identifying the mechanisms regulating ferroptosis in PCa cells is essential. Our previous study demonstrated that HJURP, an oncogene upregulated in PCa cells, plays a role in tumor proliferation. Here, we expand these findings by elucidating a novel mechanism by which HJURP inhibits sensitivity to ferroptosis inducers in PCa cells via the PRDX1/reactive oxygen species (ROS) pathway in vitro and in vivo. Mechanistically, HJURP forms disulfide-linked intermediates with PRDX1 through Cys327 and Cys457 residues. This disulfide binding promotes PRDX1 redox cycling and inhibits its hyperoxidation. As a result, HJURP enhances the peroxidase activity of PRDX1, leading to a decrease in ROS levels and subsequently suppressing lipid peroxidation induced by ferroptosis inducers. These findings reveal the potential of HJURP/PRDX1 as novel therapeutic targets and biomarkers of ferroptosis in PCa patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


