二氧化氮的电子结构
IF 1.2
4区 化学
Q4 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
利用相对论离散变化法计算了 NoO2 的电子结构和价带 X 射线光电子能谱 (XPS),能谱范围从 0 eV 到 ~40 eV。在 NoO2 中观察到了显著的共价效应,这不仅是由于 No 6d AO,还由于 No 6p 和 No 5f AO 与氧轨道的大量重叠。得出的 MO 直方图和方案有助于理解 NoO2 中价电子的化学键性质和 XPS 光谱结构的特征。本文章由计算机程序翻译,如有差异,请以英文原文为准。
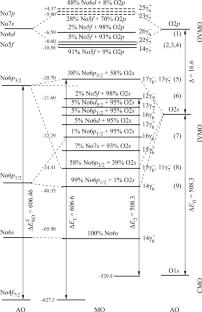
Electronic Structure of NoO2
The electronic structure of NoO2 and the valence-band X-ray photoelectron spectrum (XPS) in the energy range from 0 eV to ~40 eV are calculated by the relativistic discrete variation method. Significant covalency effects are observed in NoO2, which are due to a considerable overlap of not only No 6d AO but also No 6p and No 5f AOs with oxygen orbitals. The MO histogram and scheme are derived, which enable the comprehension of features of the chemical bond nature and the structure of the XPS spectrum of valence electrons in NoO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Structural Chemistry
化学-无机化学与核化学
CiteScore
1.60
自引率
12.50%
发文量
142
审稿时长
8.3 months
期刊介绍:
Journal is an interdisciplinary publication covering all aspects of structural chemistry, including the theory of molecular structure and chemical bond; the use of physical methods to study the electronic and spatial structure of chemical species; structural features of liquids, solutions, surfaces, supramolecular systems, nano- and solid materials; and the crystal structure of solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


