高压氧疗法作为 COVID-19 诱导的 ARDS 的免疫调节干预措施:在随机对照试验中探索临床结果和转录组特征。
IF 2.8
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
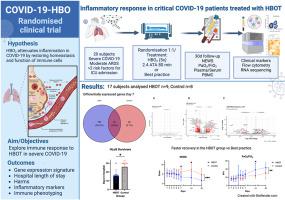
我们需要能够逆转危重症 COVID-19 的免疫调节药物,以宿主-病毒免疫反应为目标。在这项随机对照临床试验的探索性子研究中,瑞典一所大学医院的中度急性呼吸窘迫综合征 COVID-19 重症患者被随机分配(1:1)到高压氧治疗(HBOT)组加最佳治疗方法或最佳治疗方法(对照组)。随访期为 30 天。高压氧治疗在头七天内以 2.4 个绝对大气压 (ATA) 进行五次治疗,每次持续 80 分钟。对临床结果、炎症标志物和外周血单核细胞的大量 RNA 测序(RNAseq)进行了分析。在 2020 年 12 月 3 日至 2021 年 5 月 17 日期间,23 名患者接受了随机治疗,其中 17 人接受了分析。RNA测序显示,在第7天与基线相比,HBOT组有791个不同表达基因,而对照组只有46个。基因组富集分析显示,HBOT组有一个与内质网应激(ERS)相关的独特转录组特征。HBOT 组患者恢复更快,平均住院时间(HLoS)更短,为 16 天对 26 天(95.99% CI -16-0),P=0.045。HBOT组的国家预警评分(NEWS)更低(方差分析,F [8, 120] = 3.817,P < 0.001),HBOT组的PaO2/FiO2更高(混合效应模型,F [8, 94] = 2.900,P < 0.01)。我们在接受 HBOT 治疗的 COVID-19 重症患者中发现了与病毒诱导 ERS 相关的独特转录组特征。这一发现与积极的临床结果有关;与对照组相比,HBOT 患者恢复更快,HLoS 降低。试验注册:NCT04327505(2020年3月31日)和EudraCT 2020-001349-37(2020年4月24日)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hyperbaric oxygen therapy as an immunomodulatory intervention in COVID-19-induced ARDS: Exploring clinical outcomes and transcriptomic signatures in a randomised controlled trial
Immunomodulatory agents with the potential to reverse critical COVID-19, targeting host-virus immune response are needed.
In this exploratory sub study of a randomised controlled clinical trial, critical COVID-19 patients with moderate acute respiratory distress syndrome at one Swedish university hospital were randomly assigned (1:1) to hyperbaric oxygen therapy (HBOT) group plus best practice, or best practice (Control). Follow-up was 30 days. HBOT was administered with five treatments at 2.4 atm absolute (ATA), lasting 80 min, within the first seven days. Clinical outcome, inflammatory markers, and bulk RNA sequencing (RNAseq) on peripheral blood mononuclear cells were analysed.
Between December 3rd, 2020, and May 17th, 2021, 23 patients were randomised, and 17 were analysed. RNA-sequencing revealed 791 differentially expressed genes in the HBOT group compared to 46 in the control group at Day 7 vs. baseline. Gene set enrichment analysis revealed a unique transcriptomic signature associated with endoplasmic reticulum stress (ERS) in the HBOT group. Patients in the HBOT group recovered faster and had a shorter mean hospital length of stay (HLoS), 16 vs. 26 days (95.99 % CI -16-0), p = 0.045. National early warning score (NEWS) was lower in the HBOT group (ANOVA, F [8, 120] = 3.817, p < 0.001) and PaO2/FiO2 was higher in the HBOT group (Mixed effects model, F [8, 94] = 2.900, p < 0.01).
We showed a unique transcriptomic signature related to viral-induced ERS in critically ill COVID-19 patients treated with HBOT. The finding was associated with a positive clinical outcome; the HBOT patients recovered faster and had a reduced HLoS compared with controls.
Trial registration
NCT04327505 (March 31, 2020) and EudraCT 2020-001349-37 (April 24, 2020).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.20
自引率
0.00%
发文量
41
审稿时长
42 days
期刊介绍:
Pulmonary Pharmacology and Therapeutics (formerly Pulmonary Pharmacology) is concerned with lung pharmacology from molecular to clinical aspects. The subject matter encompasses the major diseases of the lung including asthma, cystic fibrosis, pulmonary circulation, ARDS, carcinoma, bronchitis, emphysema and drug delivery. Laboratory and clinical research on man and animals will be considered including studies related to chemotherapy of cancer, tuberculosis and infection. In addition to original research papers the journal will include review articles and book reviews.
Research Areas Include:
• All major diseases of the lung
• Physiology
• Pathology
• Drug delivery
• Metabolism
• Pulmonary Toxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


