瘦素通过 AKT-mTOR 信号通路促进肌腱干/祖细胞衰老。
IF 3.3
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
摘要
脂肪因子分泌失调是肌腱平衡失调的一个影响因素。高水平的血清瘦素可能是肥胖增加与肌腱病之间的潜在联系,但详细的机理解释尚不明确。在这项研究中,我们研究了瘦素在肌腱干/祖细胞(TSPCs)中的调节作用及其分子机制,并确定了高水平瘦素对肌腱恢复的影响。我们证实,瘦素以剂量依赖的方式降低了离体大鼠肌腱干/祖细胞的存活率,并伴随着转分化的增加和一系列细胞外基质(ECM)酶调制剂基因表达的改变。此外,我们还发现瘦素可以剂量依赖性地促进 TSPCs 的衰老,而在诱导细胞凋亡或自噬方面作用有限。机理研究证明,瘦素处理可增强 AKT/mTOR 信号转导活性并提高 TSPCs 中瘦素受体(LEPR)的表达,而 MAPK 或 STAT5 的激活并无明显变化。此外,我们还证实雷帕霉素治疗(而非 AKT 抑制)能有效减少瘦素促进的 TSPCs 衰老。在跟腱损伤的大鼠模型中,暴露于瘦素可显著延迟肌腱愈合,而雷帕霉素治疗可有效缓解这种延迟。我们的研究结果表明,瘦素可通过 AKT/mTOR 信号通路导致 TSPCs 细胞内在缺陷并阻碍肌腱修复。这些发现证明,瘦素水平升高在肌腱病和肌腱撕裂的治疗中起着重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
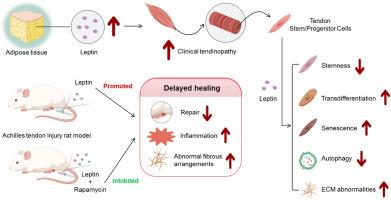
Leptin promotes tendon stem/progenitor cell senescence through the AKT-mTOR signaling pathway
Dysregulated adipokine production is an influencing factor for the homeostatic imbalance of tendons. High levels of serum leptin may be a potential link between increasing adiposity and tendinopathy, while the detailed mechanistic explanation was not well-defined. In this study, we investigated the regulatory role of leptin in the tendon stem/progenitor cells (TSPCs) and the molecular mechanism within, and determined the effect of high levels of leptin on tendon recovery. We demonstrated that leptin reduced the viability of isolated rat TSPCs in a dose-dependent way, accompanied with increased transdifferentiation and altered gene expression of a series of extracellular matrix (ECM) enzymatic modulators. Also, we found that leptin could dose-dependently promote TSPCs senescence, while exhibiting limited effect in apoptotic or autophagic induction. Mechanistic study evidenced that leptin treatment increased the AKT/mTOR signaling activity and elevated the expression of leptin receptor (LEPR) in TSPCs, without marked change in MAPK or STAT5 activation. Further, we confirmed that rapamycin treatment, but not AKT inhibition, effectively reduced the leptin-promoted TSPCs senescence. In a rat model with Achilles wounding, exposure to leptin profoundly delayed tendon healing, which was effectively rescued with rapamycin treatment. Our results suggested that leptin could cause intrinsic cellular deficits in TSPCs and impede tendon repair through the AKT/mTOR signaling pathway. These findings evidenced for an important role of elevated leptin levels in the care of tendinopathy and tendon tears.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


