单个 P450 物种功能特性的非加成性及其在饮酒对氯胺酮和阿米替林代谢影响中的表现。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
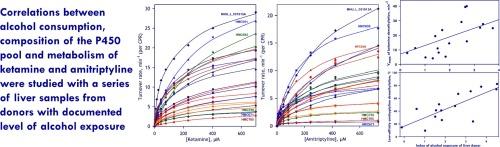
为了探索多种 P450 酶之间的功能相互联系及其在酒精诱导的药物代谢变化中的表现,我们对 P450 池的组成与阿米替林和氯胺酮去甲基化底物饱和度曲线(SSP)之间的相关性进行了高通量研究,这些底物饱和度曲线来自一系列 23 个已知有饮酒史的供体的人类肝脏微粒体制备物。SSP近似于三个Michaelis-Menten方程的线性组合,并具有全局优化的KM(底物亲和力)值。分析结果表明,氯胺酮的代谢率与酒精暴露之间存在密切联系。对于两种底物,饮酒都会显著增加低亲和力酶的作用。研究人员进一步分析了动力学成分的振幅和总代谢率与通过全局蛋白质组学评估的 11 种主要 P450 酶的丰度之间的相关性。两种底物的最大代谢率与预测的主要代谢物 CYP3A4 的丰度相关。然而,除了分别负责氯胺酮和阿米替林低亲和性代谢的 CYP2D6 和 CYP2E1 外,这两种药物的其他强代谢物都没有显示出正相关性。相反,在氯胺酮中,我们观察到与 CYP1A2、CYP2C9 和 CYP3A5 的丰度呈负相关。对于阿米替林,数据表明其对 CYP1A2 和 CYP2A6 有抑制作用。我们的研究结果表明了多种 P450 之间功能性相互作用的重要性,以及它们在酒精暴露对药物代谢的影响中所起的决定性作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Non-additivity of the functional properties of individual P450 species and its manifestation in the effects of alcohol consumption on the metabolism of ketamine and amitriptyline
To explore functional interconnections between multiple P450 enzymes and their manifestation in alcohol-induced changes in drug metabolism, we implemented a high-throughput study of correlations between the composition of the P450 pool and the substrate saturation profiles (SSP) of amitriptyline and ketamine demethylation in a series of 23 individual human liver microsomes preparations from donors with a known history of alcohol consumption. The SSPs were approximated with linear combinations of three Michaelis-Menten equations with globally optimized KM (substrate affinity) values. This analysis revealed a strong correlation between the rate of ketamine metabolism and alcohol exposure. For both substrates, alcohol consumption caused a significant increase in the role of the low-affinity enzymes. The amplitudes of the kinetic components and the total rate were further analyzed for correlations with the abundance of 11 major P450 enzymes assessed by global proteomics. The maximal rate of metabolism of both substrates correlated with the abundance of CYP3A4, their predicted principal metabolizer. However, except for CYP2D6 and CYP2E1, responsible for the low-affinity metabolism of ketamine and amitriptyline, respectively, none of the other potent metabolizers of the drugs revealed a positive correlation. Instead, in the case of ketamine, we observed negative correlations with the abundances of CYP1A2, CYP2C9, and CYP3A5. For amitriptyline, the data suggest inhibitory effects of CYP1A2 and CYP2A6. Our results demonstrate the importance of functional interactions between multiple P450 species and their decisive role in the effects of alcohol exposure on drug metabolism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


